Studying how a changing monsoon could affect our biodiversity

Scientists predict that the current bout of climate change, induced by human activities, will severely impact the monsoon. How will the expected change in this weather system affect India’s rich biological diversity? Given the complexity of both the monsoon and anthropogenic climate change, the answer is not simple.
Nonetheless, research emerging from diverse fields of biology – using both traditional and modern techniques – is providing us with valuable clues in our quest to address this critical problem. These studies are investigating how the monsoon climate, which underwent similar changes in the past, influenced the evolutionary fortunes of plants and animals in the Indian subcontinent.
The Monsoon
The South Asian summer monsoon is perhaps the most impressive meteorological spectacle on the planet. In just three months between June and September, the entire subcontinent receives three-quarters of its annual rainfall. What makes it even more dramatic is that even within this period, it is compressed into a total of just about 100 hours of copious rain.
The monsoon arrives religiously year after year. Even though we don’t always have a “normal monsoon”, it is reasonably stable over short periods of time. But this relative stability disappears as we zoom out and scrutinise it over hundreds of thousands, or even better, millions of years. Across such geological time scales, it shows up to be a fickle beast.
The monsoon is as old as the hills, literally. Its birth was triggered by the rise of a geological structure that defines the Indian landscape as much as the monsoon defines the Indian summer: the Himalaya. The Himalaya was forced into existence when the Indian tectonic plate collided violently with its larger Eurasian counterpart about 50 million years ago (mya), during a period geologists call the Eocene Epoch.
At this time, and even during much of the next Epoch – the Oligocene – the monsoon did not exist in the way we think of it today. What we had instead were weak winds which blew erratically from the Indian Ocean. It took another 25 million years or so – in the Oligocene-Miocene boundary – for the monsoon to find its feet. It was being propped up by the rising Himalaya, which was blocking the cold winds from the Arctic and northern Tibet, warming the land on the other side. The scorching hot temperatures during the summers created a low pressure area over the Indian subcontinent, drawing winds from the colder waters of the Indian Ocean. The Himalaya also helped the monsoon by serving as a barrier to the moisture-laden winds blowing from the south.
During the next 15 million years (from 25 to 10 mya), the monsoon flourished and became stronger. But this does not necessarily mean that rainfall increased. “When we talk about intensification of monsoon, what we mean is that much of monsoon winds occur during a certain period, and that’s when we get all the rainfall,” clarifies Praveen Karanth, whose lab at IISc specialises in applying molecular methods to understand how past climatic and geological events have shaped the composition and distribution of some of the subcontinent’s fauna.
By the late Miocene – about 10 mya – the landscape of India had become increasingly arid. Open dry habitats like grasslands were the new normal
Rainfall was not just being partial to certain months of the year; it was also becoming increasingly patchy in its distribution. “The intensification of the monsoon also triggered aridification of large parts of India,” says Karanth. As a result, the wet, closed subtropical forests that covered most of India started to diminish and become fragmented. By the late Miocene – about 10 mya – the landscape had become increasingly arid. Open dry habitats like grasslands were the new normal.
Both the climate and landscape of India have continued to transform past the late Miocene, but what transpired during this period has important lessons for us.
Fossils
A modified monsoon regime is likely to be upon us in the near future, if it already isn’t. Several recent studies have shown how changes in rainfall patterns could affect the ecology and behaviour of plants and animals. However, in order to understand the broader evolutionary consequences of an altered monsoon climate, it would serve us well to look back in time.
While we have been able to reconstruct the planet’s paleoclimate (using proxies which are frozen in ice, buried in sediments, or coded in tree rings), scientists are only now piecing together the story of how different groups of plants and animals – the latter being the focus of this article – responded to altered precipitation patterns.
The traditional way to address this problem would be to study fossils. For instance, in a 2015 paper, Raquel López-Antoñazas from the University of Bristol in the UK and her colleagues explored the fossil record of the subterranean Asian Bamboo Rat. These rodents, which eat roots of bamboo and other plants, are found in the eastern half of Asia, including Northeast India. But their fossils have also been recovered from many parts of North India and even Pakistan.
What the remains of the Asian Bamboo rats tell us is revealing. “Primitive” bamboo rats, which lived before about 23 mya (fossils can be aged using radiocarbon dating), had teeth that are described as brunodont and bracydont, suitable for crushing moist non-abrasive vegetation. And their skulls and skeletal structure, according to the authors, point to an above-ground lifestyle. But as the monsoon became stronger and forests disappeared, these animals were decimated. There was a massive drop in the number of species.
Then something curious happened about 10 mya – the rats made a partial comeback. The newcomers on the scene had novel dental adaptations, suitable for a more abrasive diet. Their teeth would allow them to feed by incision, followed by grinding, to eat dryer and tougher plants. The authors also claim that their skeletons reveal a subterranean lifestyle. By living in burrows, the rats could escape predators prowling the open landscape. It would also help in preventing water loss, and thus cope with the now prevalent arid environment.
Fossil evidence in other groups too highlights a turnover in species during this period. However, the utility of fossils to understand our past is limited because the odds of an organism becoming fossilised is remarkably small, says Ullasa Kodandaramaiah, an evolutionary ecologist at Indian Institute of Science Education and Research (IISER) Thiruvananthapuram. Some put this number at one in a 100 million. And that’s not surprising – only those with bones, teeth, shells, or woody stems can leave behind any remnants. Moreover, in order for an organism to become a fossil, it must be buried immediately after it dies, to ensure that its decay slows down or stops completely.
Phylogenies
Biologists have found more success in their quest to understand the evolutionary history of organisms – and how they coped with climatic changes – by building phylogenetic trees or phylogenies. “Although fossils have their own advantages, with phylogenetic methods, one has the potential to glean some information from a large number of species that exist today to understand what climatic conditions their ancestors lived in,” says Kodandaramaiah.
The logic behind such a phylogenetic reconstruction is simple: all organisms on Earth are descended from a common ancestor (Charles Darwin called it “descent with modification”). Using this assumption, phylogeneticists work backwards – they use traits of the living organisms to determine their past. The tree they build is not unlike your family tree. But whereas a family tree comprises a group of related individuals, a phylogenetic tree has related species (or sometimes populations within a species).
Traditionally, the traits that were used for such trees were typically morphological or anatomical. But since the molecular biology revolution, they have been replaced by gene sequences. Karanth spells out the reasons for this shift: “One, in molecular studies, we can generate a lot of data, and therefore get more information. Two, in molecular data, there’s a lot more variation, which you want when building phylogenies.” This is because if all the sequences are the same it would not be possible to determine how organisms are related to each other and how they diverged. And the third reason why, according to Karanth, molecular data is superior is because of something known as convergent evolution.
For example, wings have evolved in insects, bats, and birds independently (and not because they inherited it from their common ancestor). If we build a phylogeny using “wings” and other such convergent traits, we could end up with a tree which shows unrelated species being clubbed together. “An extreme example of this that comes to my mind are the vultures of the New World [Americas],” says Karanth. In the past, all vultures were grouped together, but recent DNA studies have revealed that New World and Old World vultures belong to different lineages with different ancestry. “This kind of convergence is not common in molecular sequences,” he explains.
Once phylogeneticists decide on a set of genes that are ideal for constructing a tree, they sequence them for all the species of interest. The sequence data is then subjected to probabilistic methods (increasingly based on techniques called Maximum Likelihood or Bayesian Inference) to find the tree that best depicts how this group of species evolved.
Dating Divergences
Phylogenies are more than just visual representations showing the evolutionary history of a group of species. They can also be used to understand the origins of novel traits, classify species into evolutionarily meaningful groups (clades) and test other evolutionary hypotheses. One such question a tree could be used to address is when a group of species diverged from each other and whether this divergence coincides with what we know about changes in the environment. This is done using a technique called molecular or gene clock.
“DNA sequences accumulate mutations with time. As more time elapses, there will be more changes,” Karanth says. “So if two species exhibit very high genetic distance [calculated based on the number of differences in their sequences], then one can conclude that they have diverged from each other further back in time than species pairs that show very little distance.” But this is only the first step in determining the time of divergence; it then needs to be calibrated. Though geological events are used at times for calibration, phylogeneticists typically rely on fossils. But you cannot use just about any fossil – only those closely related to the group, and can therefore be placed in the phylogeny, can be used for dating. Karanth explains the logic: “For example, if we know that there is 5% sequence difference between species A and B, and if we have a fossil dated at 5 mya that we know is the common ancestor of A and B, then the clock rate is 1% sequence difference per year.” The clock rate can then be used to determine the divergence times for other species pairs based on the degree of genetic divergence.
However, there is an important caveat. “The rate of molecular evolution may change through time and in different parts of the phylogeny, and so it is important to have as many independent calibration points as possible,” says Ishan Agarwal, an evolutionary biologist, who has studied several groups of lizards and amphibians, first during his PhD from IISc as Karanth’s student, and then as a postdoctoral research at the National Centre for Biological Sciences and eventually at Villanova University in the United States.
One group of lizards that Agarwal investigated is a genus of geckos called Cyrtopodion. It is considered palaearctic because it is thought to have come to the Indian subcontinent from the northern latitudes. But today, they are found in the arid zones of North and Northwest India. Cyrtopodion’s dated phylogenies reveal that they diversified between ten and five mya.
During this period, yet another lizard lineage that Agarwal studied, Ophisops, also experienced a similar evolutionary fate. These small grassland-dwelling lizards – there are at least 30 species including a few new ones discovered by Agarwal – dispersed into the subcontinent from the Saharo-Arabian region in the Oligocene. But the rates of diversification in this group too increased – there was an eight-fold increase in speciation rate – following the formation of grasslands in the Late Miocene.

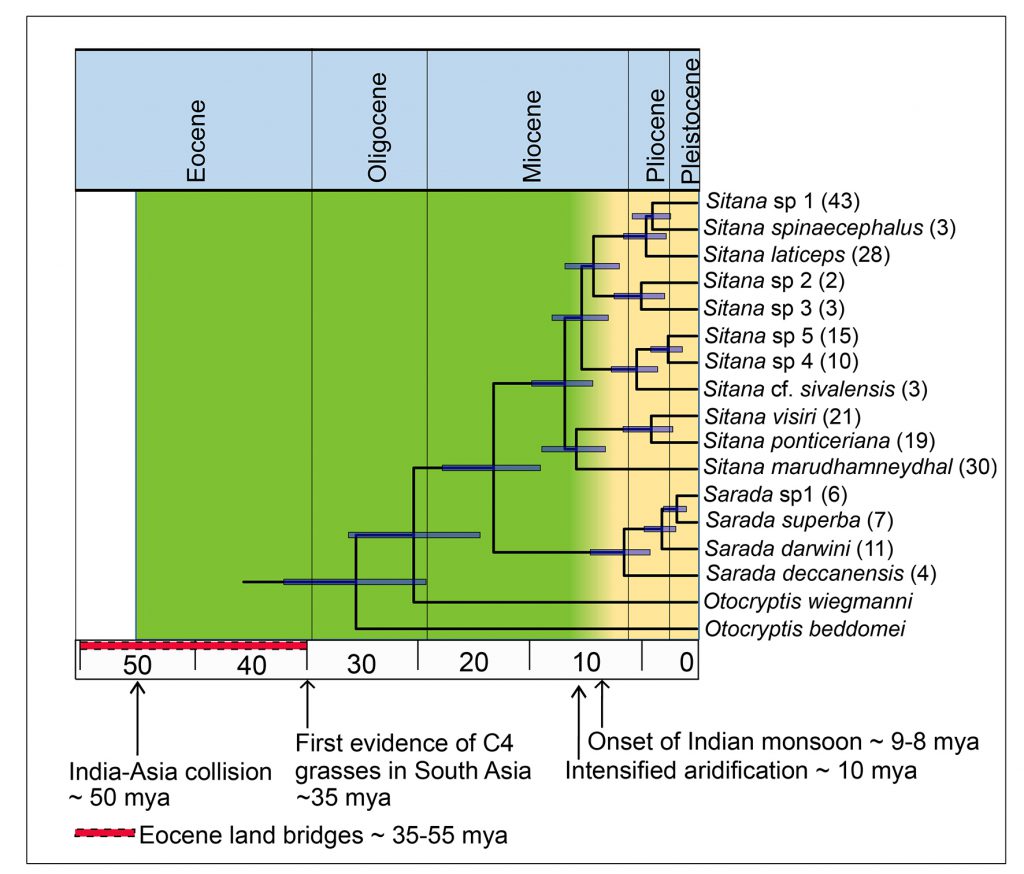
V Deepak is also an alumnus of Karanth’s lab. As a postdoctoral researcher at IISc (he is currently at the Natural History Museum, London, UK), he discovered the same story being played out in fan-throated lizards. These small, often colourful reptiles can be found scurrying across the dry landscapes of southern and eastern India, as well as Sri Lanka. There are 18 species of these lizards that are known to science belonging to two genera: Sarada and Sitana.
Deepak found that, though the group evolved about 26 mya, they underwent rapid speciation only eight to five million years ago, a period which marks the end of the Miocene and the dawn of the Pliocene. This diversification also occurred in the aftermath of intensification of the monsoon and the eventual aridification of India. What is more striking is that their closest relatives, called Otocryptis, belong to just three species, and are found in the more secluded wet forests of southwestern India and Sri Lanka. These are relics of a group of forest-dwelling lizards, which seem to have survived the age of aridification. But they didn’t have anywhere near the kind of success their close evolutionary cousins did.
In the course of their studies, Agarwal and Deepak have also described several new species – some of which do not show morphological differences (evolutionary biologists call them cryptic species). Traditional taxonomic methods, according to Deepak, have therefore severely underestimated species diversity. Their findings have other important lessons for us: grasslands and other open habitats support a healthy diversity of animal life. And many of India’s grasslands are not degraded forests; they are natural habitats that have been around for millions of years and served as fertile ground for the proliferation of some groups of animals like lizards.
“In wet-adapted species, we see lowering of diversification, which hints at extinction events, whereas the dry-adapted species underwent rapid radiation”
But not all animals were as lucky as these lizards. Those that require the humidity and protection of closed forests had seen their best days. Shield-tailed snakes belong to one such group that was adversely affected, not unlike bamboo rats. Using similar molecular techniques, these burrowing snakes, endemic to wet forests of peninsular India and Sri Lanka, were studied by Vivek Philip Cyriac from Kodandaramaiah’s lab. Once again, the study indicates a role for the prevailing climate in driving evolution: the rates at which shield-tailed snakes diversified decreased around 11 to 10 mya.
Cyriac feels that the shrinking of forests in this period could have reduced the available niches for these forest reptiles to diversify. “So it’s intuitive that the contraction of forests that started during the late Miocene would have negatively influenced diversification in forest adapted species such as uropeltid [shield-tailed] snakes.”
In their paper, Cyriac and Kodandaramaiah, however, do not invoke aridification to explain what happened in the late Miocene. They suggest that it has to do with cooler temperatures that also occurred during this period. But Kodandaramaiah points out that aridification and cooling could have gone hand in hand, and the rates at which these snakes diversified could certainly have been affected by changes in precipitation directly or indirectly. “Unfortunately, to understand the effects of past climate change, one cannot rely on controlled experiments,” he says.

A shield-tailed snake Uropeltis myhendrae in its moist habitat (Photo: Vivek Philip Cyriac)
To Karanth, the relationship between a change in the monsoon patterns and evolutionary changes is clear. “In wet-adapted species, we see lowering of diversification, which hints at extinction events, whereas the dry-adapted species underwent rapid radiation,” he summarises. Agarwal agrees: “The Late Miocene was an important time in India’s history, a time of increased aridification and habitat change – with cool- or forest-adapted groups becoming more and more restricted in distribution and warm- or open-habitat adapted groups expanding their ranges and rate of diversification.”
In spite of these takeaways, we are some ways away from a comprehensive understanding how a changing monsoon will influence the destiny of India’s flora and fauna. For one, the dated phylogenetic studies at our disposal are skewed towards certain groups, particularly herpetofauna. They need to be replicated in other animal taxa, as well as in plants. Besides, the jury is still out on how exactly the monsoon will be affected by climate change. And when we add other factors like pollution and land-use change into the mix, the challenge ahead of us is formidable.
To read more articles in our series on the monsoon, click on the following links:
Sulochana Gadgil: A Lifetime of Monsoon Research
MONTBLEX: India’s First Major Monsoon Experiment
Interview with Syed Ameenulla: ‘Ours was a small group, like one family’
BoBBLE: ‘An Unusually Successful Cruise’
What an Adivasi Village in Chhattisgarh Can Teach Us about Sustainable Development
Why Knowledge Isn’t the Barrier to Water Conservation
Chanchal Uberoi on Monsoon Melodies
Why Drain the Rivers When You Can Catch the Rain?




