A look at the human body’s sophisticated immune response to viruses like SARS-CoV-2 and why its defences are sometimes breached
A couple of weeks ago, 53-year old Prakash (not his real name) started complaining of shortness of breath. Not long after, he was diagnosed with COVID-19, the new infectious disease that has paralyzed the world. Over the next couple of days, he developed a cough and breathlessness, and his saturated oxygen level decreased.
The World Health Organisation (WHO) has described the symptoms exhibited by Prakash – besides fever, body ache, diarrhoea, sore throat and a loss of smell and taste – as being characteristic of the infection caused by the virus at the centre of the mayhem, SARS-CoV-2. First detected in Wuhan, China, in late 2019, it is thought to have originated in animals and jumped to humans, spreading rapidly across the globe. On 11 March 2020, WHO declared the disease to be a pandemic, one that has so far infected over 10 million and killed over half a million people around the world.
The invaders
Associated with respiratory illness, SARS-CoV-2 belongs to the same family of viruses that caused outbreaks of SARS in 2003 and MERS in 2012: coronaviruses. The distinctive feature of this RNA virus is the crown or corona of spike-shaped proteins on its surface. The spikes act like keys, attaching to proteins called Angiotensin-Converting Enzyme 2 (ACE2) receptors on the host cell surface. This binding allows the virus a free pass into the cells. These receptors are abundant in the cells of the lungs, intestine and heart. While the spike proteins of SARS-CoV-1 (responsible for SARS) also bind to the same receptor, the binding is much stronger in SARS-CoV-2. Researchers speculate that this is one of the primary reasons that makes this virus such a formidable adversary.
Scientists from around the world are now in a race against time to curb the spread of COVID-19, either by developing therapeutics or vaccines against the virus. But as they go about it, it is critical to understand how exactly our immune system responds to the virus.
Mounting a battle
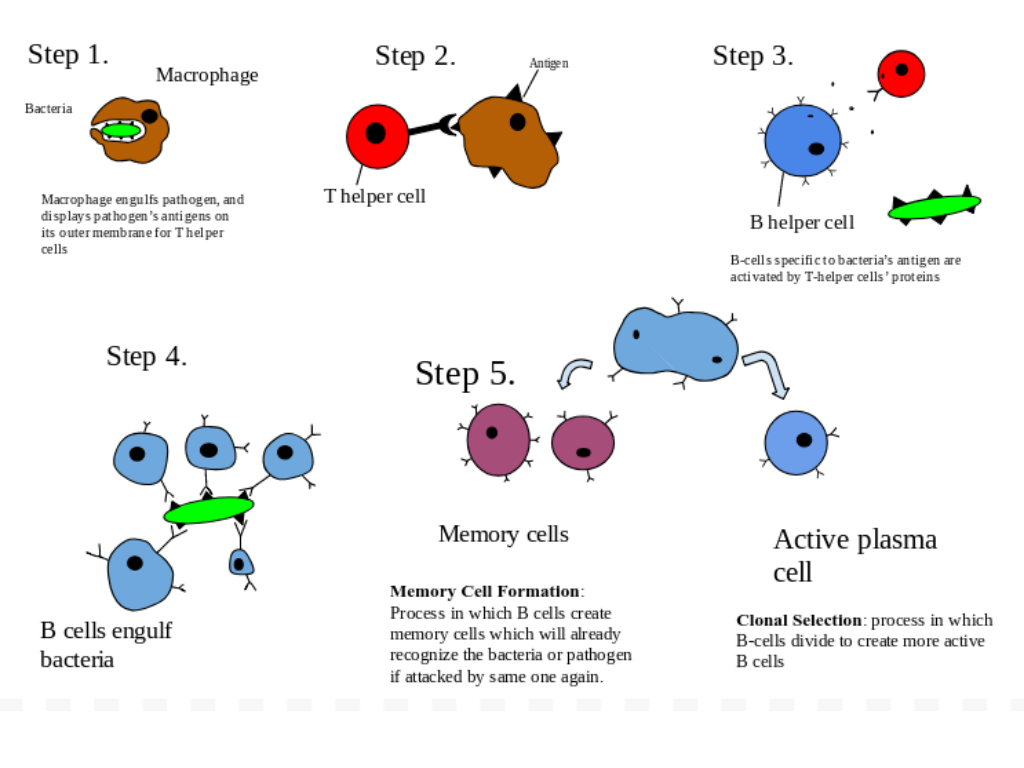
The host, in this case the human body, is not a mute spectator against the marauding invaders. It has a well-oiled immune system ready to do battle. It comprises two branches – innate and adaptive – which work together to prevent the entry of invading pathogens into the host cells, and if that does not succeed, attempt to curb their spread.
The innate immune system is the first line of defence against pathogens. It includes physical barriers, such as the skin and mucous membrane, as well as an army of foot soldiers – cells called phagocytes and NK (natural killer) lymphocytes, which help in engulfing and killing the pathogens.
If the defence mounted by the soldiers of the innate immune system is insufficient, the adaptive immune response is activated
If the defence mounted by the soldiers of the innate immune system is insufficient, the adaptive immune response is activated. Unlike the innate immune response, it has specific responses to different pathogens. The adaptive immune response involves two types of white blood cells: B lymphocytes, which produce antibodies, and T lymphocytes, which destroy infected cells. The adaptive immune system can also communicate the need for reinforcements from other immune components.
Battling the invaders
When pathogens like SARS-CoV-2 attack the body, it leads to a complex web of interactions between the components of the two branches of the immune system to recognise threats, and neutralise and “remember” them for future reference. Viruses have patterns on their surface which are recognised by specific receptors on immune cells. The main receptors that identify RNA viruses are the Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I). They induce the production of chemical signals called cytokines – which causes inflammation to help remove damaged tissues and begin the process of healing – and interferons (particularly type I) – which activate antiviral pathways. This is the rapid innate immune response at play.
Cytokine storm (Image courtesy: scientific animations.com/Creative Commons Licence/Wikimedia Commons)
It is important to keep in mind that describing the action of the innate immune system as ‘early’ or that of the adaptive immune system as ‘late’ would be an oversimplification. According to Satyajit Rath, Adjunct Professor in the Biology Department, IISER Pune, sensing by both branches occurs somewhat simultaneously, but the functional response of the adaptive immune system is delayed. “Cells of the adaptive immune system have to undergo many rounds of cell division [and maturation] so that there are enough cells to make a functional response,” he says.
The Warhorses
Viruses need the machinery of host cells to produce their proteins and to make new virus particles. But on the surface of host cells are present what are known as human leukocyte antigen (HLA) proteins. They help the immune cells differentiate between the cells of the body and foreign cells. S Vijaya, Professor at the Department of Microbiology and Cell Biology, IISc, elaborates: “When the viral antigen is made inside the cell, the short pieces of peptides resulting from the degraded molecules of viral proteins can be picked up by our own HLA and brought to the surface of the cell.”
She adds that these viral peptides on the surface of the infected cell act as a red flag for T and B lymphocytes, circulating in the blood stream carrying out routine surveillance. When alerted, a class of T cells helps in killing some of the infected cells. At the same time, antibodies on the surface of the B cells recognise the viral peptides and activate the B cells to mature and secrete a larger number of antibodies, which are more adept at recognising the viral peptides. These antibodies, called neutralising antibodies, bind to the part of the viruses that attach to the host cells.
The ability of these antibodies to neutralise viruses is the rationale behind the now well-known convalescent plasma therapy. The treatment uses the plasma of a recovered COVID-19 patient, which in principle should have antibodies against the virus. But it is employed only when patients have failed to respond to other therapeutic interventions.
Differences within the family
Among members of the coronavirus family, the RNA sequences of SARS-CoV-1 and MERS-CoV are identical to that of SARS-CoV-2 by 80 percent and 50 percent, respectively. But their antibody responses are different. “If you look at neutralising antibodies against these different viruses, they don’t match because the regions of the spike protein that recognise the human receptors are different between the virus types. The neutralising antibodies [against one virus] don’t bind [to the spike proteins of the other virus],” says Rahul Roy, Associate Professor at the Department of Chemical Engineering, IISc.
However, SARS-CoV-2 shares an interesting kinship with coronaviruses which cause the common cold, even though they are more distantly related to it (as compared to SARS-CoV-1 and MERS-CoV), says Roy. He points to a recent study by a research group from the La Jolla Institute for Immunology, which shows that in many people who have been exposed to the common cold coronaviruses, their T cells are able to recognise peptides from SARS-CoV-2. “It is not known if they can get rid of all the [SARS-CoV-2] infection, but it is predicted that they may give some protection against the virus,” he says.
The same study has also demonstrated a robust T cell response in patients who have recovered from non-severe COVID-19. While all of them showed a high response of helper T cells that initiate an antibody response, 70 percent of them made killer T cells. Discussing the implications of these two results, science writer Mitch Leslie, in Science, says, “The findings could also help researchers create better vaccines.” He makes this claim because researchers can now explore the possibility of designing vaccines that would stimulate the production of T cells.
Who is at risk?
Some people develop additional and more severe symptoms. Typically, these people tend to be over 60, or those with other underlying health conditions such as cardiac disease, lung disease, diabetes, hypertension, obesity, and chronic kidney disease. These comorbidities involve organs that can be infected easily by the virus through the ACE2 receptors.
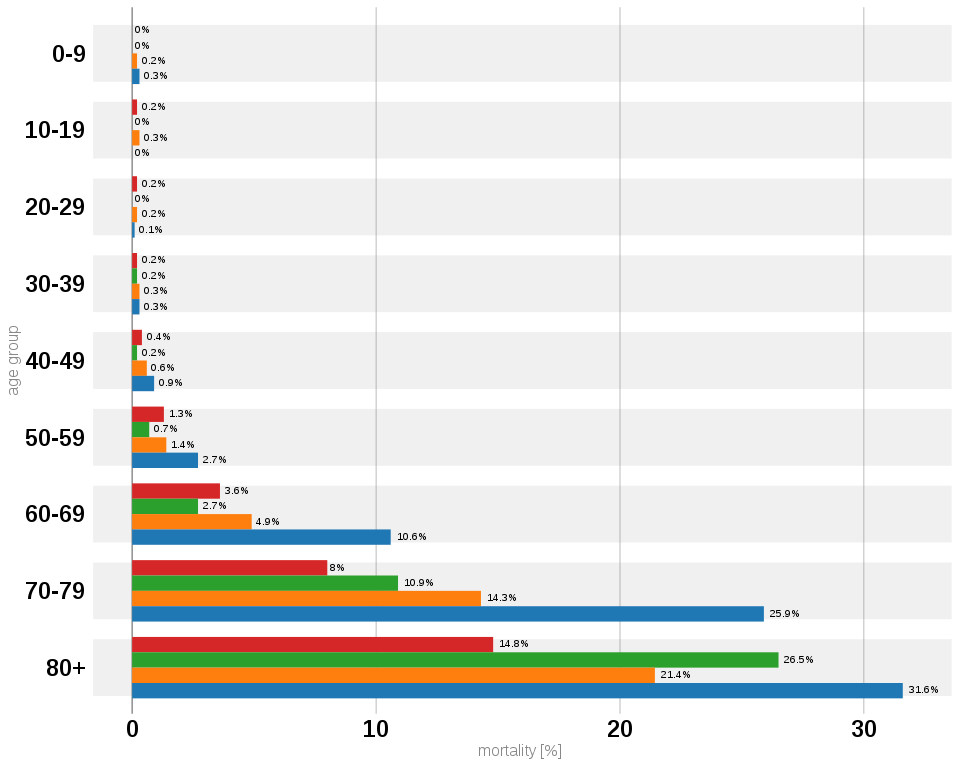
Ironically, the condition of these already vulnerable people could get worse by the action of their own immune system. While in moderate cases, signalling by cytokines results in symptoms such as fever, in severe cases, a barrage of pro-inflammatory cytokines or a cytokine storm is seen. It causes mass migration of immune cells to the lungs, where they cause inflammation, lung damage and lead to more severe symptoms, such as pneumonia and acute respiratory disorder, in addition to the regular symptoms. The cytokine storm can also affect other parts of the body like the heart, kidneys, lungs and liver.
“If a large amount of the virus got introduced into the body, or if the body’s initial response was slow to take off, the viral load will grow and there will be a large immune response,” Rath says. Using the analogy of the lockdown, he explains that the immune responses to microbes involve killing the microbes and confining them to ‘micro-containment zones’. “If and when the magnitude of the ‘containment’ of the immune response is large, tissue damage is so extensive that severe illness results.”
Prakash was among those who developed severe illness. A high risk individual – he had been diagnosed with hypertension five years ago – he soon experienced a cytokine storm, his oxygen saturation levels decreased further and so did his blood pressure. His case had progressed from ‘moderate’ to ‘severe’ within a span of eight days.
Ironically, the condition of these already vulnerable people could get worse by the action of their own immune system
As part of his treatment, Prakash was put on ventilator support to ensure adequate oxygen supply to his lungs. He was administered steroids to stop the cytokine storm and other drugs to prevent complications like clotting in the blood vessels of the lungs and possible infections by other pathogens. Fortunately for him, the treatment worked. The intensity of his symptoms reduced and he was taken off ventilator support. Prakash is now stable and on the road to recovery.
The asymptomatic riddle
Another piece of the puzzle that researchers are still trying to understand is why so many COVID-19 patients do not show any visible symptoms. A recent study on asymptomatic patients by a research group in China showed that these people shed viruses for a longer period than symptomatic patients, and also had a lower inflammatory response. But curiously, their antibodies against SARS-CoV-2 fade more quickly than their symptomatic counterparts. There could be two possible reasons for this, Roy speculates. “Either they did not develop enough immune response and hence might be susceptible to new infections, or their ‘less-pronounced’ immune response was enough to suppress serious COVID-19 symptoms.” But it is still not clear as to how susceptible recovered asymptomatic people are to future infections.
Danger of reinfection
A few viral infections like dengue are more severe when they reinfect a recovered patient. This is due to a phenomenon called antibody dependent immune enhancement. According to Vijaya, viruses bind to the antibodies that were made against them during the first infection. These antibodies, whose job is to destroy the virus, instead act like a Trojan horse and help the viruses gain access to the host cells, which they would normally find difficult to infect.
Some researchers have suggested that this antibody dependent immune enhancement could be a possible reason as to why the severity of COVID-19 varies among people belonging to certain age groups and regions. They attribute it to their differing levels of exposure to other coronaviruses, which share similarities in the spike proteins with SARS-CoV-2. Roy adds that this enhancement could also have implications for the development of COVID-19 vaccines, since a vaccine should not cause enhanced entry of viruses in case of a future infection.
We have come a long way in understanding how our immune system defends our body against foreign agents. But scientists are still in the process of investigating the many dimensions of its response to novel pathogens like SARS-CoV-2. Their research will be crucial to the success of the global endeavour to overcome the pandemic. But until then, we need to take all the precautions that we know will help save lives and slow the spread of the virus: limit exposure of those who are vulnerable to the virus, practice physical distancing, and ensure personal hygiene.
Anoushka Dasgupta, a science writing intern at the Office of Communications in IISc, is a Master’s student in Biotechnology from Savitribai Phule Pune University, Pune
For more stories about the COVID-19 crisis, click on the links below:
Tracking the Scourge: Diagnostics, Testing and Vaccines for COVID-19
Engineering Our Way Out of the COVID-19 Crisis
The Pandemic and Mental Health at IISc
Understanding COVID-19 Through the Lens of the Social Sciences




