An in-depth look into biological tools and techniques being developed at IISc in the fight against COVID-19

As the country went into lockdown in March 2020, researchers at IISc turned their focus to joining the fight against the novel coronavirus, SARS CoV-2. This article delves deep into ongoing research in diagnosing and testing for COVID-19, as well as a project on vaccine development, one of the few such efforts being undertaken in the country.
The search for a vaccine
The word “vaccine” has its origins in the Latin word for the cowpox-derived injection used to treat smallpox by Edward Jenner in the 18th century. Since then, vaccines have come a long way, and currently, there are several different types in use.
“In India, the vast majority of vaccines that have been produced have been based on the relatively old technology of taking the inactivated pathogen, such as many of our childhood vaccines. Or they have taken vaccines which have been clinically tested elsewhere, and then manufactured here,” says Raghavan Varadarajan, Professor at the Molecular Biophysics Unit. “There have been very few fully indigenous vaccines”.
“There have been very few fully indigenous vaccines”
For a deadly disease such as COVID-19, developing vaccines rapidly is especially critical. There are currently 149 vaccine candidates being developed across the world.
One such candidate is a recombinant protein subunit vaccine for COVID-19 being developed in Varadarajan’s lab. Subunit vaccines use a fragment of the infectious microorganism to induce a strong, targeted immune response. In this case, these are fragments of the spike glycoprotein of SARS-CoV-2 expressed in cell cultures of other organisms such as bacteria or yeast. Animal immunisation trials that measure the antibody response to the candidate vaccine have been undertaken in collaboration with Mynvax, an IISc-incubated start-up.
Although a majority of COVID-19 vaccine research across the world is focused on a relatively new modality called mRNA vaccines, Varadarajan says that technology has not yet been deployed on a large scale. “Whereas there is [and we have] a lot of experience with using protein subunit vaccines. Therefore this was our approach of choice.” It will take at least 12-18 months before their subunit vaccine can go into clinical trials. In the meantime, Mynvax is in active discussions with several manufacturers for commercial production.
Antibody testing
Vaccines induce our body to produce antibodies. Similarly, antibodies are also generated when we get infected with the virus. Detecting these antibodies can give important insights into the spread of a disease that other diagnostic methods cannot. A kit to detect antibodies for COVID-19 is now being developed in Rahul Roy’s lab at the Department of Chemical Engineering. These antibodies can be detected in the body a few weeks after a person is infected.
“These antibody tests are not proof that someone is immune, but most of the time they are highly correlated with immunity. So if you test positive for the [presence of] antibodies, it is quite likely that you are also immune to the disease,” says Roy.
Such antibody tests have several applications. Health agencies and the government can use them to quantify the prevalence of a disease in a given population. They can also be used to decide who needs to be vaccinated, for recruiting volunteers for plasma therapy, and for measuring the success of vaccine candidates in clinical trials. In the context of the novel coronavirus, the use of such antibody tests as “immunity passports” has also been the subject of much debate.
Roy’s lab is collaborating with the Bangalore Medical College and Research Institute (BMCRI) and St. John’s Medical Institute, as well as with Deepak Saini, Professor at the Department of Molecular Reproduction, Development and Genetics (MRDG), IISc. The team is in talks with companies for manufacturing the kit. To ensure the safety of the researchers working on this project, the lab only deals with samples (provided by the BMCRI) of patients who were initially infected with COVID-19, but have tested negative twice after discharge. These blood samples would contain the antibodies, but would no longer be infectious.
According to Roy, choosing the right antigens ‒ parts of the virus that are recognized by our immune system ‒ is crucial to making a successful kit. He adds that they have taken a non-conventional approach in choosing small peptides as well as entire proteins. Since protein quality can vary from batch to batch, and it also degrades over time, these peptide-based antibody kits will have a longer shelf life, with better quality control. Roy expects their kits to cost only about Rs.100, enabling them to be deployed on a large scale.
The antigens were designed in collaboration with the Tata Institute of Fundamental Research (TIFR), Hyderabad, using computational methods that allow one to predict which parts of the viral protein would give rise to immune response. The group chose combinations of peptides that are genetically conserved (and do not undergo mutations) but at the same time do not match with any other coronaviruses, such as those that cause the common cold. They believe that their antibody test should therefore be able to detect immune responses specific to all the strains of SARS-CoV-2. “A good serology test should have high specificity and sensitivity, that is, it should be able to detect the presence of COVID-19 all the time, but should never detect any other disease,” says Roy.
Faster and cheaper tests
The current gold standard for COVID-19 diagnosis is RT-PCR (Reverse Transcriptase Polymerase Chain Reaction), a technique that looks for the virus’s RNA, but it can take hours to display results. A few research projects at IISc are trying to reduce this turnaround time and the costs involved, and to enable testing to be scaled up in remote areas.
A few research projects at IISc are trying to reduce this turnaround time and the costs involved, and to enable testing to be scaled up in remote areas
Azooka Life Sciences, another start-up from IISc, has successfully launched RNAWrapr, a viral transport medium that does not need refrigeration, thereby greatly reducing the cost of transporting samples. RNAWrapr also inactivates the virus to some extent, reducing the risk of infection from handling the samples, and protects the RNA from degradation. Azooka has already supplied 1,000 units to the Shankara Cancer Hospital in Bangalore, a designated sample collection centre for COVID-19, and other distributors have also shown an interest in this product.

RNA extraction takes around three hours to complete, and RT-PCR facilities are available at only a few hospitals and labs. Azooka is also developing a point-of-care diagnostic kit that does not need RT-PCR facilities or trained medical personnel in a laboratory, and produces results in 30 to 90 minutes. It uses LAMP (Loop mediated isothermal amplification) which does not require a thermal cycler that is typically needed for PCR. The final result can be loaded onto a lateral flow device, and visualised as bands on a dipstick (as positive or negative), similar to a home pregnancy test. Azooka has partnered with BMCRI to develop and validate this kit using COVID-19 samples.
Although Azooka had support from the Society for Innovation and Development at IISc when they were incubated, they faced hurdles in kick-starting work on their kit as they struggled to get access to lab space within the Institute. However, they were eventually able to ramp up their efforts on their own.
“Ideally when we develop these kinds of diagnostic kits, it takes at least 3-6 months for validation and to test for reproducibility and performance,” says Fathima Benazir, co-founder of Azooka and a former postdoc at IISc. “Otherwise when people rush to develop a test and put it in the market, it fails in the user environment.”
Similar efforts are ongoing in Bhushan Toley’s lab at the Department of Chemical Engineering to develop a paper-based diagnostic kit for SARS-CoV-2, by replacing PCR with a LAMP assay, but with all components fitted within a specially engineered strip of paper. They are considering several different readout methods to detect and view the results – colorimetric detection, fluorescence probes and a lateral flow assay. The LAMP assay has previously been tested successfully in their lab for detecting tuberculosis.
The RT-PCR test also requires liquid reagents. As an alternative, Toley’s lab is trying to develop dry reagents for these kits that are premixed, and need to only be rehydrated before use.
Lab-on-wheels
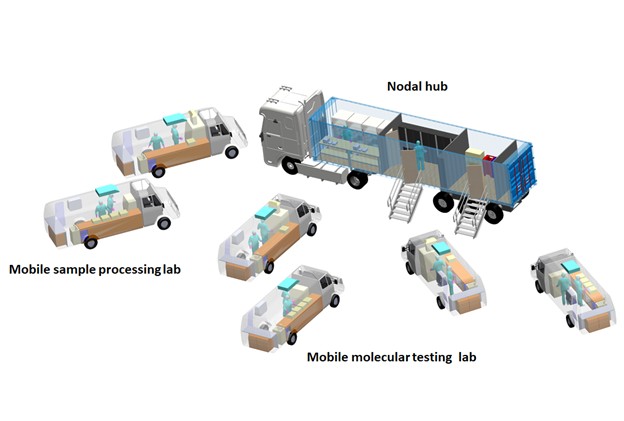
One of the major bottlenecks to scale up testing for COVID-19 is the lack of a large number of labs with Bio Safety Level-2 (BSL-2) or higher safety levels, and trained personnel required to handle them. To overcome this, Sai Siva Gorthi’s lab at the Department of Instrumentation and Applied Physics has designed a fleet of mobile laboratories.
These consist of a large container truck that acts as a nodal hub and warehouse facility, and several other smaller vans that have been converted into satellite labs. The vans would be equipped to perform different operations ‒ one with BSL-2 facilities for sample collection and inactivation, another for viral RNA extraction and isolation, and a third for conducting the diagnostic RT-PCR tests. The nodal hub would have storage facilities for consumables and a workbench, as well as facilities for bio-waste disposal. The hub would be parked at a location within a COVID-19 infected district, while the satellite labs travel around and collect and analyse samples, coming back to the hub at the end of the day. This project has been undertaken in collaboration with ShanMukha Innovations, another IISc incubated start-up, and BMCRI. The mobile labs are ready to be deployed and will soon be handed over to the state government.
COVID-19 (Image courtesy: Sai Siva Gorthi’s lab)
Gorthi is also collaborating with Toley’s lab for developing a point-of-care RT-PCR testing technology for enabling rapid and cost-effective testing of infectious diseases. Their Masters student, Rajas Poorna, has demonstrated a proof of concept for a handheld and energy-efficient ultrafast PCR device based on an optical heating mechanism. In future, this technology would be used in the mobile labs as well.
Setting up a COVID-19 test facility at IISc
Efforts to set up a test facility for COVID-19 at the Institute started towards the end of March 2020. This turned out to be a timely move, because soon after, a directive was issued by the Ministry of Human Resource Development (MHRD) to all the institutions funded by the central government to set up diagnostic facilities for the novel coronavirus.
The test centre was set up under the direction of Umesh Varshney, Chair, Division of Biological Sciences, and spearheaded by faculty members Shashank Tripathi (Centre for Infectious Diseases Research or CIDR), Amit Singh (Department of Microbiology and Cell Biology) and Deepak Saini (MRDG), with guidance from Vijay Chandru at Strand Life Sciences and Dr V Ravi at the National Institute of Mental Health and Neurosciences (NIMHANS). “Setting up a diagnostic lab, logistically, is a very complex process, not as easy as it sounds…because there is no room for error here, as somebody’s life is at stake,” says Tripathi. “We have done it in a miraculously short time, in a span of three to four weeks, despite the lockdown, due to tremendous support from top-down at the Institute.”
Housed at the Bio Safety Level-3 (BSL-3) facility in one of the labs at CIDR, the actual diagnostics at the test centre are handled by a team of hired professionals, including a few former postdoctoral fellows. Varshney clarifies that no IISc PhD students work at the test centre. The working group consists of three teams – one that handles samples in the containment facility and deactivates them, another which processes the non-infectious samples to get RNA for PCR, and a third that deals with data management. The personnel work in shifts, and rotate between the three teams to ensure that fatigue and monotony do not set in, and errors are avoided. Packaged meals were provided to the test centre by the Institute mess even during the lockdown.

Saini points out that the personnel who work at the test centre were hired with informed consent, and must undergo basic health check-ups at the Health Centre as well as an intense two week orientation and training in the Standard Operating Procedures (SOPs) for working with SARS-CoV-2 samples. They also need to pass an evaluation before they can begin. These SOPs were formulated along guidelines issued by the Indian Council for Medical Research (ICMR), the World Health Organisation (WHO) and the Centres for Disease Control and Prevention (CDC). The training is imparted by R S Rajamani, a research staff member who has managed the BSL-3 facility at CIDR for many years now.
Varshney and Saini say that there has been tremendous support from the Karnataka State Government and the ICMR, which provide RT-PCR kits and personal protective equipment (PPE) for the test centre.
“Diagnostics is very different from what we usually do in day-to-day research, and the initial one or two weeks went into training staff, developing the SOPs, and getting them cleared by the Institutional COVID-19 and Biosafety Committees, as well as by the nodal ICMR officer,” says Singh.
Once test results are obtained, they need to be reported in several different formats to different authorities, such as the ICMR, the state government, the District Surveillance Officer, and to the hospital they came from. The test centre also needs to maintain inventories of reagents being consumed, of how many samples were received, their barcodes, and the details of personnel involved at every stage of testing. All this generates a huge amount of data, which they initially struggled to manage. Now, these operations have been automated using a tool called Redmine (an open source web-based project management tool), with help from G-KnowMe Consulting and the IISc IT team. “The reports can be sent to the different authorities with a single click of the mouse, and the data can be backed up, sorted and filtered as needed,” says Tripathi.
As the lockdown eased and COVID-19 cases began rising again, there was an urgent need to scale up testing in Karnataka. From 600 tests in mid-May, the IISc centre has performed close to 9,000 tests by 30 June 2020.
From 600 tests in mid-May, the IISc centre has performed close to 9,000 tests by 30 June 2020
Singh says that the long term vision is to generate a repository of COVID-19 samples and build resources that can be explored for academic purposes. He hopes that the Institute continues to support the facility for at least another six months to a year, and that the immense societal contribution of the personnel and the faculty members working at the test centre is recognised.
Varshney concurs. “The idea was that if we set up this facility, currently we will be helping a social cause, and in the future, having such a facility will help us with infectious diseases research in the Institute.”
With inputs from Ranjini Raghunath
For more stories about the COVID-19 crisis, click on the links below:
Engineering Our Way Out of the COVID-19 Crisis
The Pandemic and Mental Health at IISc
Understanding COVID-19 Through the Lens of the Social Sciences




