Why urban diseases like rabies and dengue are so hard to control
Less than a kilometre from Bengaluru’s Lalbagh botanical garden lies the Bisilu Maramma Devi temple. The goddess has another name: Plague Maramma. Devotees believe that the deity harnesses the sun’s power to kill bacteria and viruses and ward off illnesses.
The temple is one of several that people flocked to when a devastating plague struck the city in 1898. Driven by congestion and poor sanitation, the disease went on a rampage, killing hundreds and prompting a mass exodus. Riots broke out over the stringent measures that the ruling British government took to screen and isolate patients. Distrust of “foreign” medicine prevented many Indians from taking life-saving plague inoculations.
The outbreak also led to a revamp of Bengaluru’s civic infrastructure. A citywide telephone network was set up to coordinate plague control measures. New healthcare centres, including the Victoria Hospital, were built. Old buildings were demolished en masse in congested areas, and better lit and ventilated houses were constructed. Citizens were encouraged to move to newer layouts like Malleswaram and Basavanagudi, which had wide roads, modern drainage networks, and plenty of greenery.
Today, however, that modernised city is reeling from repeated outbreaks of vector-borne and zoonotic diseases. It is a major hotspot for dengue fever in Karnataka, accounting for nearly half of the state’s reported cases. Rabies deaths in cities like Bengaluru and Delhi have ratcheted up each year – India alone accounts for 36% of the world’s total rabies deaths, according to the WHO.
“Cities are a more saturated interface between animals and people,” says Abi Vanak, Senior Fellow and Director, Centre for Policy Design, Ashoka Trust for Research in Ecology and the Environment (ATREE). This proximity to potential disease vectors, in addition to rapid urbanisation, overcrowding, and improper waste management, has made city dwellers more vulnerable to infectious diseases.
Disease spread in urban areas depends on several ecological, environmental, and socio-economic factors. Viruses that cause diseases like dengue and rabies constantly evolve to evade human interventions. Vaccine development and distribution are stymied by scientific and logistical challenges. Vector surveillance and control measures are generally ill-planned and post-hoc. All of these have created a complex cocktail of conditions that allow largely preventable diseases to not just persist but also thrive in urban areas.
“Many of these diseases are not just healthcare problems but also socio-economic problems,” says Utpal Tatu, Professor in the Department of Biochemistry, IISc. “They most commonly affect people from lower economic strata.”
“The way people live, the way we are reshaping the environment around us so fast … does have an effect on diseases and their vectors,” says Farah Ishtiaq, Principal Scientist at the Tata Institute for Genetics and Society (TIGS). Dengue fever, for example, was once a forest disease that spilled over to humans due to increased deforestation. “The mosquito and virus have become so acclimated to the urban environment that we think that they have always been around us,” she adds.

The origins of the dengue virus are murky. Scientists think that it was circulating in primates in the jungles of either Africa or Southeast Asia about 1,000 years ago, and jumped to humans a few hundred years ago. Hitching a ride on Aedes aegypti – a tiny black-and-white striped mosquito – the virus zoomed across the globe, before making inroads into Asia after World War II.
The lifecycle of the dengue virus alternates between the mosquito and the human. When a female mosquito bites a human already carrying the dengue virus, the latter enters and multiplies inside the mosquito’s salivary glands. The next person that this virus-laden mosquito bites then gets injected with the virus, continuing the chain of transmission.
Once the virus enters our bloodstream, it hijacks our cells and multiplies, setting off alarms in our immune system. Our body responds by attacking the virus and turning up the heat (“dengue fever”). Sometimes the immune system may overreact, unleashing a storm of inflammatory signals leading to plasma leakage from blood vessels, organ failure, and even death.
“The problem with dengue is that the causative agent is not just one virus,” says Rahul Roy, Associate Professor in the Department of Chemical Engineering, IISc. “It’s a large group of related viruses that infect in roughly the same way.”
There are currently four serotypes (variants) of dengue circulating in India. Based on genomic analysis, Rahul’s team has shown that Dengue 1 and 3 serotypes were dominant until 2012, but Dengue 2 is currently the most common serotype across the country. Dengue 4, once thought of as the least infectious, has become the predominant south Indian strain.
The virus also mutates remarkably fast. “It can mutate even within an individual host. Sometimes, the virus that infects you is not the same virus that later grows in your body. It is even capable of adapting to different tissues in our body,” Rahul says.
Rahul points out how the same virus that thrives inside a mosquito is able to survive inside a human with such vastly different physiology. “You have such a pliable organism, a chimera that can change itself so quickly,” he explains. “It is challenging to develop a drug or vaccine because the pathogen does not remain the same. A new variant of the pathogen will come – maybe even out of the intervention that you did.”
When did zoonotic diseases first emerge?
Analysis of ancient human DNA suggests that they started hopping from animals to humans at least 6,500 years ago, and that farming and livestock domestication sped up their spread. The plague caused by Yersinia pestis, for instance, seems to have emerged about 5,000 years ago when farming communities in the Eurasian Steppe started domesticating livestock. When these pastoralists later migrated rapidly across Europe and Asia, along with their languages and genes, they may have also unleashed the plague among local populations, much like how colonial diseases wreaked havoc on Native American populations in North America.

The rabies virus has an unusual modus operandi. When it enters the human body – usually through a bite from an infected animal – it attacks muscle cells, sneaks into neurons via neuromuscular junctions, and then travels along the nerves up to the brain. One of its key targets is the limbic system – a part of the brain that regulates emotions and behaviour.
The stealth invasion of the central nervous system is one of several tricks that the virus uses to evade elimination. In the initial stages, it multiplies slowly while silently suppressing chemical signals that could alert immune cells. Once it reaches the brain, it gets shielded from circulating immune cells by the blood-brain barrier.
Infected victims first experience mild flu-like symptoms – fever, discomfort, headaches. As the disease progresses, they start to feel intense pain, excess salivation, anxiety, aggression, and a fear of water. In the final stages, their neurological functions rapidly deteriorate, leading almost certainly to death. By the time it reaches the brain, it is “100%” fatal, says Utpal.
“Rabies is a very complex disease,” he adds. For several years, Utpal’s team has been working on understanding the different clades (lineages) of the rabies virus. They have been able to identify at least four different clades and pinpoint specific mutations present in each. This can help track or develop interventions against each clade independently. His team has also developed a PCR-based assay to identify the presence of two of the main clades.
“It is one of the oldest known diseases,” Utpal adds. “But there is a lack of information on the different variants, their pathogenesis, and outcomes.” Scientists believe that the rabies virus first spread in bats thousands of years ago and later evolved to infect larger mammals like dogs. Its ability to inhabit a wide variety of mammalian hosts has allowed it to persist for centuries. As dogs’ proximity to humans – either as pets or strays – grew, so did opportunities for the virus to spread in urban areas.
One of the quirks about rabies, says Abi, is its unpredictability. The incubation period – the time between infection and appearance of symptoms – can vary widely, from a week to a year. “A dog that’s infected today may not show symptoms for months,” he says. Some animals, like the African yellow mongoose, have been known to survive with rabies, without visible symptoms, for years.
Symptoms may also vary from animal to animal and may be mistaken for other diseases. “Most people only recognise the classic ‘furious’ form of rabies – dogs attacking and biting people,” Abi adds. “But there are lots of rabies cases in which the dog doesn’t do anything. It might look like it has a distemper virus or a head injury, or it might just appear lethargic. That is equally dangerous.”

In 2016, the Philippines launched a large vaccination campaign to give Dengvaxia, a dengue vaccine developed by the pharma company Sanofi Pasteur, to 1 million schoolchildren. It was a disaster. Children without a prior dengue infection faced an increased risk of hospitalisation or severe symptoms if they caught dengue after vaccination. After 19 children died, the campaign was suspended.
Dengue poses an unusual problem. When a person gets infected by a specific dengue serotype, our immune system creates antibodies that latch onto the virus and neutralise it. These antibodies offer protection for a few years. But if the same person catches a new infection from a different serotype later, the older antibodies are unable to fully eliminate the newcomer, but they can still bind to the virus and inadvertently smuggle it into our immune cells. Inside the immune cells, the new serotype goes on a rampage, multiplying rapidly, resulting in a more severe infection.
In the Dengvaxia case, the jab acted like the first infection, triggering a more severe second infection. Following the fallout, the WHO recommended that the vaccine should only be given to people who either live in places with an extremely high number of dengue cases – where benefits outweigh risks – or to those who already have a diagnosed past infection.
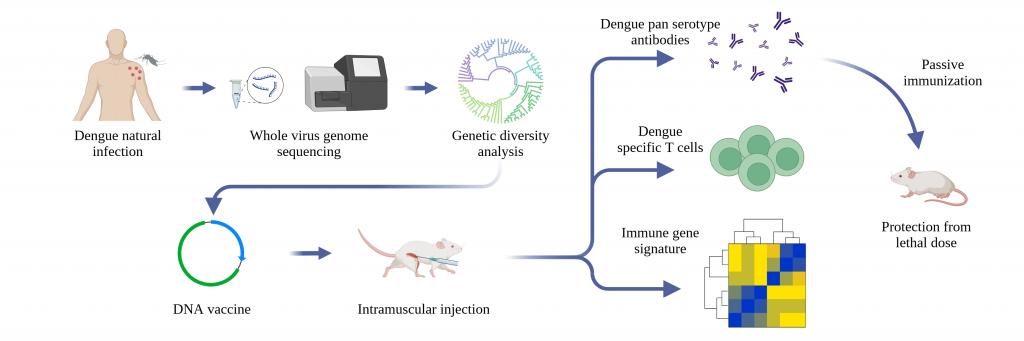
But it’s hard to screen for past infections because most people don’t show symptoms. Some estimates put the level of dengue antibodies in urban populations at 70-80%, according to Rahul, meaning that most people have already had a dengue infection at least once in their lifetime.
The fact that all four serotypes circulate contemporaneously in India also boosts the chance that one can get infected by any one of them at any time.
“You might see a spike in dengue one year and then the next two years you might not see dengue cases as much in your locality,” says Rahul. After a few years, when antibody levels against the first infection start dropping, a new serotype moves in, changing itself to mimic the older virus just enough to evade immune detection, and slowly establishing itself as the new dominant strain.
“This complicates the development of a vaccine, because you need one that generates antibodies against all dengue serotypes and possibly other new variants that might arise,” says Rahul. “People have been working on such dengue vaccines for 20-30 years now.” So far, most dengue vaccines have only helped stave off serious symptoms, not prevent infection entirely. Rahul speculates that vaccines currently approved for use abroad also might not work well in India because the local serotypes are different from the strains used to develop those vaccines.
Rahul is currently part of a multi-institutional effort to develop a DNA-based vaccine for dengue that can protect against all four serotypes. “Studies in mice are promising,” he says. “Human trials have to be carried out.”
What makes bats the most efficient viral vectors?
During the COVID-19 pandemic, conspiracy theorists falsely blamed its emergence on people eating bat soup. Bats have also been painted as villains because of their link to frequent Nipah virus outbreaks. But bats are indeed pretty good at hosting some of the deadliest viruses without dying or showing symptoms. One theory is that as they evolved to fly, their metabolic rates and body temperatures shot up, which can damage DNA, forcing the animals to develop robust DNA repair mechanisms that also help them fight off viral infections. The fact that they roost together in close-knit communities also encourages viral exchange and adaptation. But most viruses remain within these bat communities and only spill over to humans when there is habitat destruction or rare instances of close contact.

On 6 July 1885, a nine-year-old boy who had been bitten by a rabid dog 14 times was rushed to the lab of French scientist Louis Pasteur. Pasteur had been experimenting with a crude vaccine that he had developed from sun-dried spinal cord sections of rabbits killed by rabies. He administered the vaccine continuously for 13 days and the boy survived. Pasteur’s vaccine formulation continued to be used, with some modifications, until the early 20th century.
Modern rabies vaccines for humans contain the inactivated virus grown in animal cell lines and can help prevent as well as treat the disease. Post-exposure doses need to be given immediately after an animal bite, along with a shot of rabies immunoglobulin – a medication containing anti-rabies antibodies. “The extent of disease depends on how much the virus has multiplied in the dog, the degree of bite, the depth of the wound, and the bite location – close to the hand or neck or elsewhere,” explains PN Rangarajan, Professor in the Department of Biochemistry, IISc. “As soon as a dog bites, if you immediately wash it with soap water and take the vaccine shot, the disease can be prevented. You have to basically catch the virus before it enters the nervous system.”
But rabies vaccines may not always be available immediately, especially at underserved public health centres.

“The current human rabies vaccine has to be lyophilised [freeze-dried for storage and transportation]. It is very expensive to make,” says Rangarajan. “There are probably only three or four companies that are making it in the country.” Several years ago, Rangarajan and his team tried to develop a DNA rabies vaccine that would not require cold storage and significantly cut down costs. “We got a patent and published studies and even tied up with a pharma company to manufacture it. Toxicology studies were carried out in mice and monkeys. The Department of Biotechnology came forward and agreed to fund regulatory trials under the Jai Vigyan Vaccine Mission,” he says. “But it did not move further.”
Pet and stray dogs also need to be regularly given doses of veterinary rabies vaccines. “You have to conduct vaccinations at very high levels to maintain herd immunity in dogs,” says Abi. “But Bengaluru has about 4-5 lakh stray dogs; you can’t vaccinate all of them in one go.”
Vaccination is also only one component of rabies control, points out Rangarajan. “We need to have a multi-factorial approach.”

A factor that’s often overlooked in dealing with zoonotic diseases is understanding the life cycle and behaviour of vectors. In India, surveillance of disease vectors is almost non-existent, says Farah. “We are not looking at the ecology of vectors like mosquitoes at all.”
Farah’s team has been tracking A. aegypti, the primary dengue vector. “Aedes mosquitoes don’t need a water-filled habitat to lay eggs. They just need shallow water or even a damp surface. When it rains, their eggs hatch and you have lots of mosquitoes.” There is also a misconception that dengue mosquitoes only breed in dirty water, she adds.

Tracking mosquito numbers can help map dengue hotspots. Farah’s team has been placing ovitraps – mesh-covered containers with water that attract female mosquitoes to lay eggs – which can help measure egg densities across the city. This can serve as a proxy for mosquito numbers. “We’ve also been trying to design sticky traps – they mimic an ovitrap but the lining is kind of gluey, so when the female lays eggs, it also gets stuck to the side wall,” she explains. Counting these adult female mosquitoes – which are the main carriers – can also give an idea of potential dengue incidence. “You can also screen the [trapped] adult mosquitoes for other viruses like the one that causes chikungunya.” This data can be matched with past clinical data from fever clinics or hospitals to pinpoint hotspots.
Identifying these hotspots early can help municipal authorities plan ahead, says Bhaskar Rajakumar, Programme Director of Healthcare Initiatives at ARTPARK, IISc. For the last few years, he and collaborators at IISc and other institutes have been working with the Bruhat Bengaluru Mahanagara Palike (BBMP) to develop a digital dashboard that can predict which areas might face a dengue surge. “They asked us to come up with predictions so that they can make policy decisions,” he explains. “How many paracetamol tablets should they stock? How many beds should they keep reserved? Do they need to send more healthcare staff to certain areas?”
To create the dashboard, the ARTPARK team first obtained line list data – how many patients reported fever and in which areas – from government records over 8-9 years. Then they pooled in climate data – temperature, humidity, rainfall – as well as mosquito breeding spot surveys and photos taken by ASHA workers. They fed all of this to machine learning models and created a digital dashboard showing which regions in the city are likely to face increased dengue cases. “In disease or climate modeling, people usually use a single model. We tried an ensemble approach using seven different models,” Bhaskar explains. “As of now, we can provide a two-week advance prediction at the sub-district level, with about 70% accuracy.”
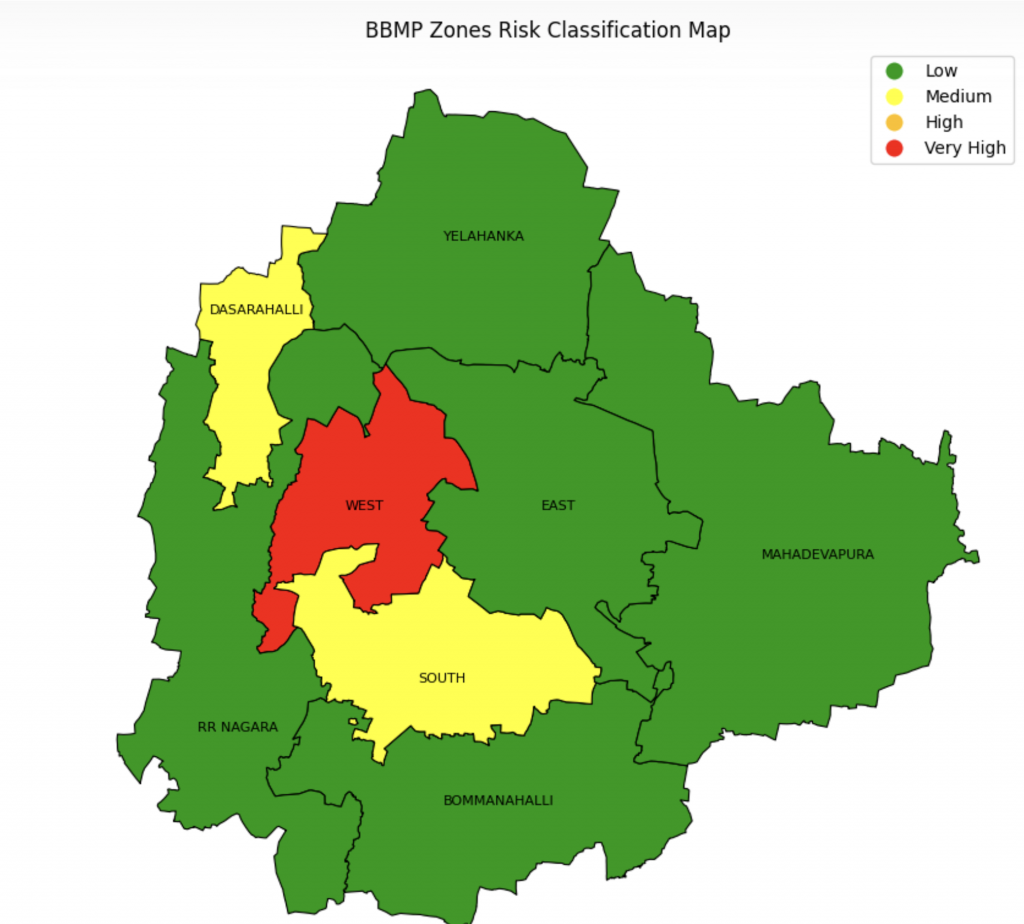
Karnataka – BBMP areas at zone level (Image courtesy: Bhaskar Rajakumar)
Last year, when dengue cases hit a new high, some of these predictions helped BBMP take necessary measures, Bhaskar adds. “They set up temporary fever clinics. They could also mobilise ASHA workers and field health workers to look for and clear out breeding spots.”
Could a zoonotic disease ever lead to a zombie apocalypse?
If yes, rabies might be the best contender. Some manifestations of the disease, like aggressive behaviour and transmission via bites, already match pop culture depictions of a zombie outbreak – think Resident Evil or 28 Days Later. The chances of the rabies virus being maliciously modified into a bioweapon are extremely remote, but what if the virus naturally adapts to reduce its incubation period inside a host in order to spread faster? Or what if at some point it becomes aerosolised like the flu virus? Fortunately, these possibilities lie firmly in the realm of science fiction. For now.

“India appears to be the world capital of rabies,” Utpal says. “It has the highest number of human deaths due to dog bites and rabies transmission. The numbers on government websites are probably underestimates, as many cases go unreported.”
In August 2025, after a six-year-old child died from rabies following a stray dog bite, the Supreme Court passed a sweeping decision to round up thousands of stray dogs from the streets of Delhi and lock them up in shelters, a move that re-ignited an age-old debate about animal versus human safety on city streets. It also shows how responses to spikes in disease numbers are often knee-jerk reactions. Animal activists, for example, have long advocated for large-scale vaccination and sterilisation as more humane and proactive solutions.
“Most cities in India don’t have rabies control, technically speaking,” Abi says. “They will carry out sterilisation and vaccinations at a small level, but the numbers [of dogs vaccinated] are so small compared to the actual population that it’s virtually useless.”
A few years ago, Abi and collaborators worked with a non-profit animal hospital in Pune called ResQ to carry out rabies surveillance. They set up a helpline for people to call in and report suspected rabies cases. Animals were brought into ResQ and tested for rabies using lateral flow assays. “Out of 1,200 dogs, at least 750 were positive for rabies. 40 of them were pet dogs,” he says. “A lot of these animals did not show any classical symptoms of rabies.” Based on the data, the Pune Municipal Corporation vaccinated about 23,000 dogs. The team also distributed educational material about rabies prevention to people across the city.
Abi and others have also carried out studies to analyse dog feeding patterns in cities. “Most people who feed street dogs don’t take any responsibility for them – they don’t take them for vaccination or sterilisation,” he says.
Beyond vector control, a bigger issue is seeing these animals as isolated factors rather than part of an interconnected system that encompasses human, animal, and environmental health, a concept referred to as the ‘One Health’ framework. “Any dialogue related to zoonotic diseases usually only involves veterinarians and doctors,” Abi says. “It doesn’t involve environmental scientists, hydrologists, ecologists or even social scientists – human behaviour [too] is an important determinant of disease emergence.”

In 2024, when dengue cases in the state hit a new high, the Karnataka government declared the disease an epidemic and imposed fines ranging from Rs 400 on households to Rs 1,400 on construction sites if there was any water stagnation.
Simple steps like clearing away stagnant water or preventing garbage from piling up on streets can go a long way towards keeping disease vectors at bay. But many people don’t follow such simple rules, point out Farah and Rangarajan.
“Rabies has been eradicated in Western countries. In the USA, you will never see a stray dog. If you have a pet dog, you need to have it on a leash, and scoop up its poop,” points out Rangarajan. “We want to follow these countries in designing metros and missiles. We should also follow them in enforcing such simple rules, no?”
Even if awareness is not a major issue, attitudes can be, Farah adds. “BBMP does a good job of communicating guidelines about dengue in both English and Kannada,” she says. “If you go to the heart of the city, you will find ASHA workers doing a brilliant job of educating people about vaccination, clearing breeding spots, and so on. If you go to an affluent apartment complex, people won’t even let you inside or listen to you – but they’ll have 10 plant pots with stagnant water that may be breeding mosquitoes.”
Reducing the burden of urban diseases is not impossible. Bengaluru once used to be a city of wells and lakes that offered prime locations for the malaria mosquito to breed. But when people switched to groundwater and piped water supply, malaria cases dropped drastically, Farah explains.
“If we can eradicate a disease like polio, why can’t we eradicate a disease like rabies?” Rangarajan asks. “It all depends on the government’s resolve, and the cooperation of NGOs, public health agencies, and the public.”
(Edited by Abinaya Kalyanasundaram)




