The consequences of leaving females out of biological studies
Abha Khandelwal vividly remembers some of her unusual cardiac cases, a lot of them women. Like the 35-year-old woman who was watching her son play football when she suddenly felt nauseated. The mother brushed it off, wanting to stay and watch her son’s game. But her son felt that something was off and encouraged her to go to the emergency room.
There, the doctors realised that she was having a less-known type of heart attack — one where the artery tears and restricts blood flow. This was unlike normal cases, where plaque buildup clogs the artery. When the doctors questioned her further, she eventually mentioned feeling a mild chest pressure. Chest pain or pressure is usually the first symptom that doctors hear about during a heart attack, but not in her case.
“I’ve dedicated my career to taking care of heart disease in women,” says Abha, Associate Professor of cardiovascular medicine and Cardiac Director of the maternal heart program at Stanford University. “But it’s very hard to take good care of [patients], when we don’t have good science and data supporting our interventions.”
Despite women comprising half the world’s population, sometimes doctors do not fully understand how to treat them. Be it heart disease, diabetes, or dementia – it is becoming increasingly clear that women experience many diseases differently from men and also respond differently to treatments. But clinical data is still lacking due to complex biological and social reasons. Women are often not enrolled in clinical trials as much as men, and in many cases, scientists opt to use male mice models instead of females to do the preclinical experiments. As a result, the same drugs can have highly variable outcomes and side-effects in women compared to men.
Despite women comprising half the world’s population, sometimes doctors do not fully understand how to treat them
“In the US, historically, we have been applying whatever the current [clinical trial] data is to both sexes. But if you look at where the data is originating, it’s often from middle-aged Caucasian males,” Abha explains.
Scientists have known that fluctuating female hormones – like estrogen and progesterone – could affect female physiology, making it harder sometimes to interpret clinical trial data in women. “There may be, therefore, a preference to use male subjects in clinical trials,” says Sandhya Visweswariah, Honorary Professor in the Department of Developmental Biology and Genetics (DBG), IISc. “It’s just that when you’re testing something, it is better to test it in a system in which the variables are less.”
The Thalidomide tragedy in the early 1960s further complicated matters. Women in many countries used to take a drug called Thalidomide for morning sickness, but it led to birth defects in many babies. This prompted the USA-based Food and Drug Administration (FDA) to enforce rigorous testing of any new drugs before they are approved. In 1977, as the rules for clinical trials were being shaped, the FDA decided to bar women of child-bearing age from participating in Phase I and II clinical trials, because of potential risks to unborn babies.
But this exclusion eventually led to almost all women being left out of clinical trials.
Only in 1986 did the National Institutes of Health (NIH) in the USA start reconsidering this strict ban, realising that doctors needed to fully understand women’s biology to treat their illnesses. And in 1993, the FDA issued a revised guideline stating that sex differences must be evaluated in clinical drug trials.
Despite the revised guidelines, even if some studies enroll more women, many of them still do not seem to provide a sex-specific analysis of the results. And in spite of multiple diseases being highly prevalent in women, they continue to be under-represented in many studies.

Symptoms and side-effects
Another reason for fewer women in clinical studies could be social challenges. “Women are generally the caretakers and will sacrifice their own health for the society around them,” says Abha. “So, if you’re creating a study that requires multiple site visits and long hours, and they are responsible for ageing parents, children and spouses, they are not going to do it.”
For example, when it comes to heart disease, women have a dizzying range of atypical symptoms, which complicates diagnosis. “Sometimes it’s hard to really get the history from some of my female patients,” says Abha. “Men will [simply] say: ‘I’m having chest pain.’ Whereas women might have a more exhaustive list of symptoms – of which one is chest pain. But it can get lost in all the other symptoms.’”
In India, a study across 17 hospitals between 2011 and 2015 found that even if women had higher comorbidities, they were not given the correct treatment for their cardiovascular issues.
What causes heart attacks in women can also be different. Instead of the typical plaque build-up in the big arteries – called atherosclerosis –women can have spasms or tears in the arteries. Sometimes, instead of large arteries getting blocked, small blood vessels can be troublesome for women.
“When we were studying heart attacks back in the 1960s and 70s, it was men going to work and dying of a heart attack. So [clinicians] developed the angiogram, where they looked at the big arteries, because that’s what was happening to the population they saw dying,” explains Abha. “At the time, the field was not aware of the impact of the disease on women and how additional testing is sometimes required.” She and her colleagues recently published a review on how increasing the number of women in cardiovascular clinical trials can help achieve better outcomes for women. “It is a call to action for all women; if we want better healthcare for our hearts, we need to participate in the science studying and treating it,” she says.
Another consequence of limited clinical trial data on women is that how they react to drugs is not well known. A 2020 UC Berkeley study showed that since most drugs were designed based on men, women might be overmedicated, leading to increased adverse side effects. Indians largely rely on generic drugs because they are cheaper. But there are a lot of clinical symptoms and variations seen in women after taking these drugs, according to Nikhil Gandasi, Assistant Professor at DBG, IISc.
For Nikhil, the problem hit close to home. Both his parents-in-law take metformin, a common drug prescribed for diabetes. When they get their HbA1c levels – a measure of blood glucose – tested, the results vary. “With my father-in-law, it’s more under control, below six,” he says. “With my mother-in-law, even though she takes those [same] drugs, she doesn’t get her HbA1c levels lower than seven.”
This is one piece of anecdotal evidence, but in some cases, the problem might be widespread – a 2017 Uruguay-based study found that sex-based differences in gastrointestinal physiology can influence how generic drugs are absorbed by men versus women. And similar to the situation in cardiovascular diseases, women of child-bearing potential have been unnecessarily excluded from type 2 diabetes clinical trials as well, according to a 2016 review in Diabetes Care.
The disparity also extends to disorders of the brain. Alzheimer’s disease (AD) is known to be almost twice as common in women than in men, with studies also linking menopause to increased risk for AD. Yet, women are understudied. According to a 2022 UK-based study, the number of women taking part in dementia clinical trials is better than in other diseases (58%), but it does not match the proportion of women in the global dementia population (64%). Plus, 113 out of the 118 trials analysed didn’t report sex-based differences. “It appears that these trials are focused on treating one sex, as the mechanisms driving the brains of each sex are different,” says Smitha Karunakaran, Assistant Professor at the Center for Brain Research, IISc.
Another consequence of limited clinical trial data on women is that how they react to drugs is not well known
Take the case of a newly approved AD drug called lecanemab. Phase III clinical trials produced stronger effects in men than in women, with men having a 43% slowing of cognitive decline versus women who had only 12%. “It’s likely that males are benefiting more from this drug than females are,” study author Madhav Thambisetty, senior investigator at the National Institute on Aging, USA, tells Axios.
Lecanemab is also known to have side effects called amyloid-related imaging abnormalities (ARIAs) – essentially brain swelling and bleeding. But how intense and frequent these are in men versus women is not fully clear. A key scientist involved in the lecanemab research recently gave a talk at IISc on how the drug leads to fewer ARIAs compared to others. Someone in the audience asked: “What are the male/female differences with ARIAs?” The scientist replied: “Oh, that’s a wonderful question. We did not check it.”
Model systems
Another reason why drugs might have varied effects in women is that even preclinical trials and lab research are skewed towards males. In many cases, scientists prefer to use male mice, as females go through a complex hormonal cycle called the estrous cycle. Many scientists believe that this cycle could make experimental results in females harder to interpret.
“We know that hormones affect behaviour. So, when you’re doing behavioural sciences, you want things to stay the same,” says Sandhya.
Annaliese K Beery, Associate Professor at the Integrative Biology and Neuroscience Department at UC Berkeley, however, claims differently. A tireless campaigner for the inclusion of female mice in biological research, Annaliese cites evidence that female mice are not inherently more variable than males at any stage of their estrous cycle. Be it gene expression, hormone levels, or even behaviours related to fear and anxiety, there were actually cases of males having higher variability.
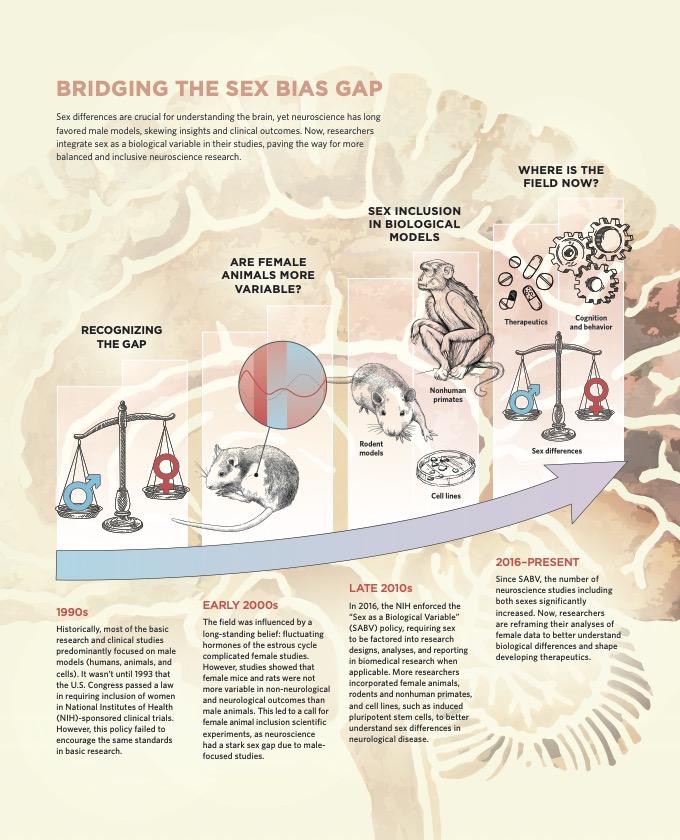
From article: XX marks the spot: Addressing Sex Bias in Neuroscience
Li Gan, Professor in neuroscience at Weill Cornell Medicine, New York, is also a strong advocate for using both sexes in AD research. She has written about how sex hormones and sex chromosomes lead to differences between male and female mouse brains – especially during ageing – recommending that sufficient sample sizes of both sexes should be used to design drugs more carefully.
In 2016, NIH announced a “sex as a biological variable” research policy, mandating that biological sex should be considered whilst designing experiments and reporting results. Journals have also become more strict and ask researchers to specify the sex of the animal models used, sometimes even encouraging them to repeat their experiments on female animals. This has gradually led to more labs looking at both sexes in studies.
Smitha’s lab in CBR is one of them. Her team has seen that female mice show symptoms of impaired memory at a much older age compared to male ones, suggesting some sort of protective mechanism at work in female brains. Her lab is now trying to figure out these mechanisms. Another recent study from multiple labs at CBR has shown that although AD is more prevalent in women, they show some degree of cognitive resilience – their decline can sometimes be slower compared to men.
The gut is another point of difference. Sandhya’s lab has found that mutations in a receptor called guanylyl cyclase C lead to severe gastrointestinal disease in humans. However, certain stressors to the gut caused male mice with the mutation to show more severe reactions than female mice.
“The gut has a very tight barrier to prevent the entry of harmful material into the body,” says Sandhya. But in some genetically modified mice, the barrier was leakier in male mice. “So with that observation, we decided to look very closely at the differences between the male and the female gut.”
Equal participation
Be it increasing the use of female mice in research or improving the numbers of women in clinical trials, strong voices amongst scientists and clinicians are now pushing for change. Abha points to a study that showed how having more women in selection committees for large-scale clinical trials resulted in more women enrolled.
Some scientists are also advocating for responsible and safe inclusion of pregnant women in clinical trials. The American College of Obstetrics and Gynaecology prefers to call pregnant women “scientifically complex” rather than “vulnerable”, and in a significant advance in 2019, these women were no longer categorised as a vulnerable population by the FDA.
Abha’s 35-year-old patient – whose nausea signaled a heart attack – eventually recovered fully. However, many women may not be as lucky until female biology is better understood. Raising awareness and outreach for women to take part in trials, and for scientists to report sex-specific analyses from clinical studies, is essential. “In all of the clinical trials I conduct, even if it costs more and takes longer, I will always enroll at least 50/50 [male and female]. I don’t think it’s fair to do it any other way,” Abha says. “Further, as women continue to focus their efforts on the health and wellness of their family, they must realise that it is imperative to start with themselves.”
(Edited by Ranjini Raghunath, Abinaya
Kalyanasundaram)




