How its understanding has changed through the years
The year was 1919. May was approaching and so was graduation season at the University of California, Berkeley in the USA. A young undergraduate student was in deep distress. One of his professors, William Bray, had assigned a paper to be submitted before the end of the term. But the student had not written anything at all.
As a last resort, he approached Bray with an idea. Some of his instructors had earlier discussed ‘unsolved problems of chemistry’ in their classes, and they intrigued him. He had the reckless idea of just submitting his crude notes of possible solutions for some of those problems.
The student was Maurice Huggins. Among those solutions he presented in the paper was the seed for what later evolved into the concept of the hydrogen bond: he proposed that the hydrogen atom could perhaps be bonded to two other atoms in some organic compounds.
Huggins didn’t himself use the term “hydrogen bond”. In 1920, chemists Wendell M Latimer and Worth H Rodebush working in the same university expanded on Huggins’ suggestion in a paper, in which they wrote: “Mr Huggins of this laboratory, in some work as yet unpublished, has used the idea of a hydrogen kernel [atom] held between two atoms as a theory in regard to certain organic compounds.”
Many years later, in a 1936 paper, Huggins still only used the term “hydrogen bridge”. He thought that the word “bond” only referred to a system having one or more electrons bringing two atoms together. He also felt that calling it a bond might confuse people into thinking that it was a connection between two hydrogen atoms and not between hydrogen atom and the atom of another element.
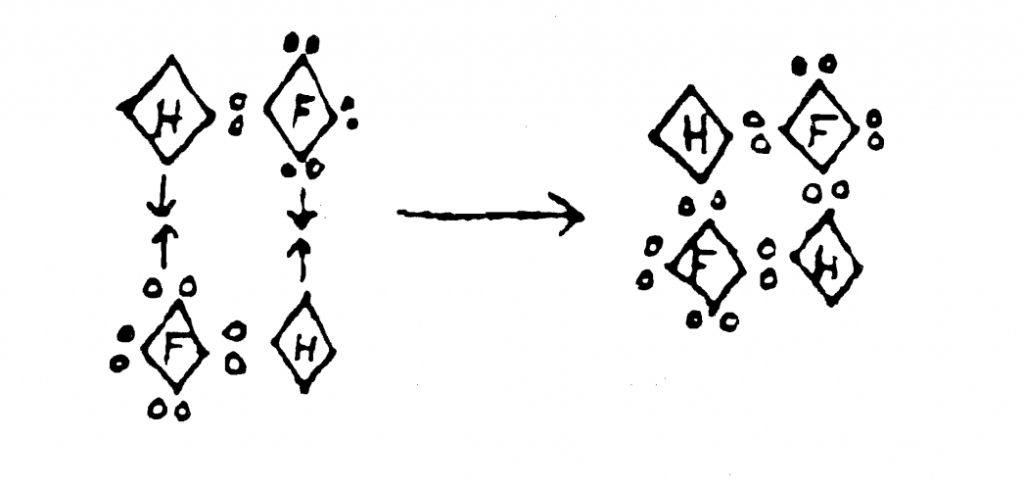
showing a hydrogen-bonded hydrogen fluoride dimer (Image courtesy: Bulletin
for the History of Chemistry 1990/ACS)
Huggins was right to have his reservations at that time. In fact, Bray told him that he would never get the chemistry world to believe that such a bond could exist. By then, valency – a measure of how many bonds an atom can form – had been discovered. It was common knowledge that hydrogen was monovalent, meaning that it had only one electron revolving around its nucleus to spare for bond formation. A bond (covalent or ionic) usually needs at least two electrons, one each from the participating atom.
“It was a very strange idea at that time,” explains Himansu Sekhar Biswal, Professor at the School of Chemical Sciences at NISER, Bhubaneswar. “The idea of it bridging between two atoms, essentially acting as a “bigamist” as the British chemist Henry Armstrong later sarcastically put it in 1926, seemed completely contradictory to the known rules of bonding.”
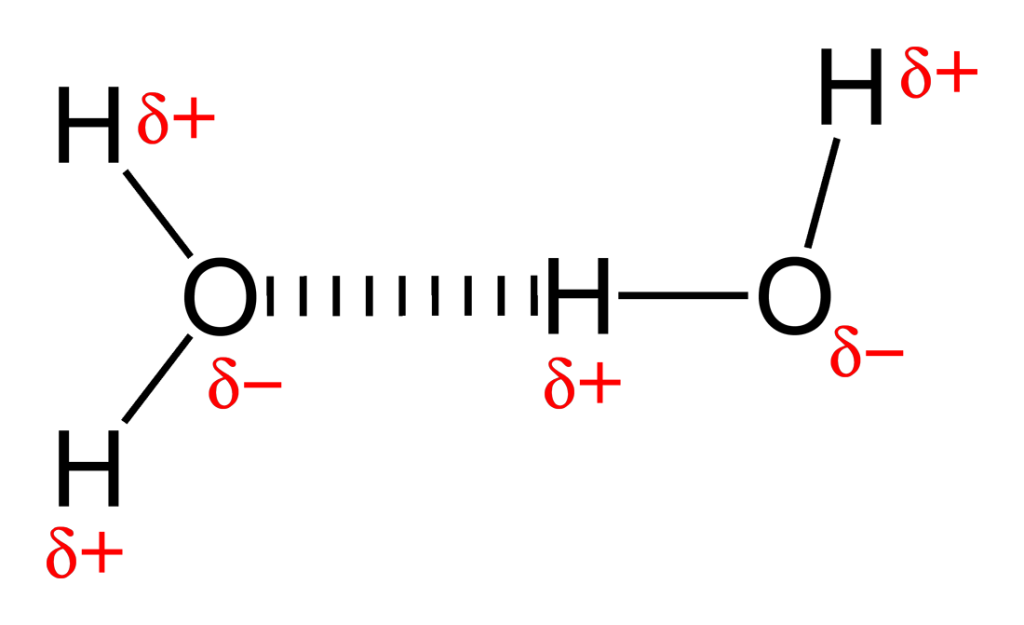
What exactly is a hydrogen bond? When a hydrogen atom forms a covalent bond – in which electrons are shared between atoms – with an electronegative (electron-attracting) atom, the former attains some “extra” positive charge. This is because the electronegative atom is able to pull the shared pair of electrons towards itself, causing the bonded hydrogen atom to develop a partial positive charge. This extra charge allows it to interact with one more electronegative atom in the same or different molecule. Of course, the nature of this interaction is still not fully clear.
Hesitation to call it the hydrogen bond persisted till the 2000s. In a popular textbook published in 1999, former IISc faculty member Gautam Desiraju and Thomas Steiner, former researcher at Freie Universität Berlin, still referred to it only as the “weak hydrogen bond”.
Given its mysterious nature, scientists spent a lot of time scrutinising it. Linus Pauling, the most influential chemist of the 20th century, initially declared hydrogen bonds to be electrostatic, meaning that the atoms are able to attract or repel electrons by virtue of electrical charge. But this view waned gradually, as more and more studies revealed that it was not a mere electrostatic interaction.
‘Given its mysterious nature, scientists spent a lot of time scrutinising the hydrogen bond’
Scientists thought that maybe the hydrogen bond was formed from dipole-dipole interaction. When two oppositely charged entities – like the hydrogen atom with a partial positive charge and the electronegative atom with a partial negative charge – are separated by a distance (forming a ‘dipole’), interaction can arise between two such dipoles. Thus, a dipole-dipole interaction is essentially a form of electrostatic interaction. Typically, the direction of a dipole is indicated from positive charge to negative charge.
Then, scientists looked closely at the structure of a hydrogen fluoride (HF) molecule linked via hydrogen bonding to another HF molecule – called a dimer. If the HF molecules behaved like dipoles, they should have interacted in one of two directions – parallel or antiparallel. In other words, if it were a dipole-dipole interaction, it would assume one of two configurations, either a straight line or a “square”.
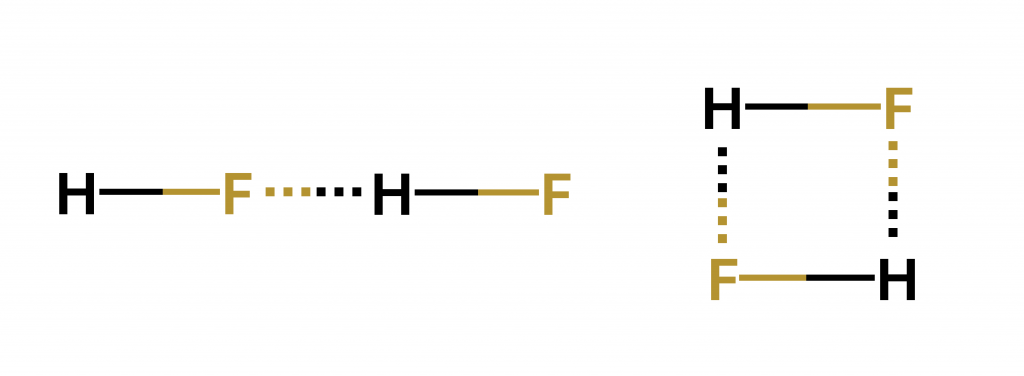
But after the development of advanced spectroscopic techniques, the HF dimer structure turned out to be completely different from what scientists had envisioned – more like a step-ladder.
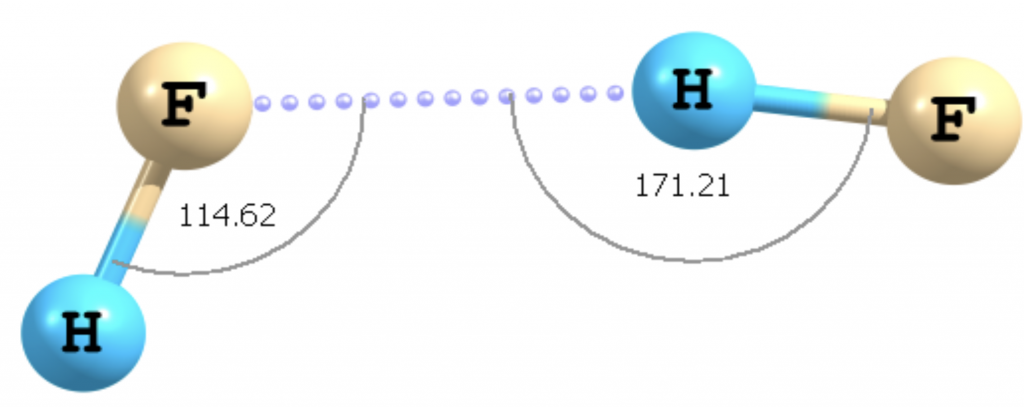
Clearly, the hydrogen bond was not solely a dipole-dipole interaction. After almost a century of research, scientists now widely believe that a hydrogen bond is not a single entity, but a complex interplay of different factors like dipole-dipole interactions, charge transfer, orbital interactions, and other phenomena. Some say that it may perhaps have a bit of covalent character too.
“The hydrogen bond depends on physical parameters, like distance between the donor and acceptor atoms, and the angles involving the three atoms participating in the bond,” remarks Suryaprakash Nagarajarao, former Professor and Chair, NMR Research Centre, IISc.
Hydrogen bond everywhere
Should we care about the hydrogen bond? Some scientists certainly think so. In 2025 alone, about 17,000 papers on the topic have been uploaded on Google Scholar so far.
“Life would simply not exist if not for the hydrogen bond. Everything would evaporate, become inanimate matter,” says Suryaprakash.
For example, protein structures are held together primarily by a variety of interactions, including the hydrogen bond.
The bond grew in popularity after scientists discovered its role in supporting DNA structure. DNA consists of two nucleotide strands linked to each other through hydrogen bonds, to form a double helix. While an individual hydrogen bond by itself is not very strong, it is the sheer number of bonds between the strands which greatly stabilises DNA.
Another example of how hydrogen bond affects chemical properties of molecules is the stark difference in boiling points of compounds called hydrides formed from elements in a specific group of the periodic table. The boiling points of hydrides are expected to follow certain trends, usually increasing as you go down the table, since the atomic mass increases in that direction. But that is not the case for water (H2O), hydrogen fluoride (HF) and ammonia (NH3). Because they have hydrogen bonding, they have dramatically high boiling points compared to hydrides of their neighbouring elements.
Hydrogen bonding is also the underlying reason for the difference in boiling points of some compounds that have the same mass. Take ethanol and methoxymethane, for example. Both have the same mass of 46u (atomic mass unit). But while the boiling point of ethanol is 351K, the boiling point of methoxymethane is 249K. This is due to the intermolecular hydrogen bonding present in ethanol but not in methoxymethane.
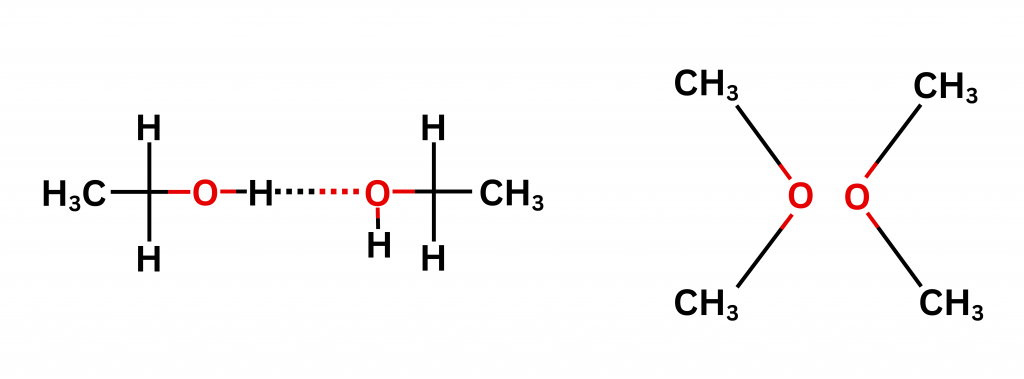
Suryaprakash’s lab has used Nuclear Magnetic Resonance (NMR) to prove the participation of organic fluorine in the hydrogen bond. This is impor tant because around 30% of the commercially available drugs contain at least one fluorine atom. The intra- and inter-molecular hydrogen bonds play a dominant role in determining the structure and conformation of the drug molecules.
Although the importance of hydrogen bonding quickly became clear to scientists, its definition took a long time to gain acceptance.
Defining the bond
When scientists initially proposed that the hydrogen bond was a weak electrostatic interaction between a hydrogen atom bonded to a highly electronegative atom, the definition felt incorrect as it did not quite seem to capture the essence of the bond. One reason was because many believed that only some electronegative atoms oxygen (O), nitrogen (N) or fluoride (F) could interact with hydrogen in the expected manner.
“It was believed that less electronegative atoms, like sulphur and selenium, could perhaps form very weak hydrogen bonds, but they weren’t considered significant players,” explains Himansu. The electronegativity value of sulphur was lower than the electronegativity values of N, O and F. Scientists, therefore, believed that only strong electronegative atoms like the latter could participate in hydrogen bonding.
That changed when people started investigating the crystalline structure of H2O (water or ice) and H2S (hydrogen sulphide).
Let’s take a step back and look at these two. Chemists consider ice as the ideal hydrogen bonding system. In solid state, each water molecule is bonded to four other water molecules by hydrogen bonds. This makes sense, because each hydrogen atom in a water molecule can get bonded to one water molecule each, and the oxygen atom in a water molecule can accept hydrogen bonds from a hydrogen atom of two other water molecules.
But H2S, with a similar formula to H2O, does not have the same crystalline structure as ice. Scientists found that H2S forms a closely packed cr ystalline structure, like the one in ionic solids. An individual molecule of H2S is surrounded by twelve other H2S molecules.
Pauling ascribed the structure of ice to the presence of the hydrogen bond and that of H2S to van der Waals interaction. Because he was – and is – a prominent figure in the chemistry world, this distinction went without repudiation for a long time.
When E Arunan, Professor at the Department of Inorganic and Physical Chemistry (IPC), IISc, and his team set out to investigate this, they found something interesting. The structure of a H2S dimer at ultra low temperatures, when observed in a pulsed nozzle Fourier transform microwave spectrometer, resembled that of ice. “We had to go to temperatures way past its freezing point to unveil this,” he remembers.
So, why had the tightly packed structure been seen before? At the freezing point of H2S, the thermal energy of the system is still enough for the molecule to ‘rotate’ and appear like a circle – like a ceiling fan. The true structure of a fan is three blades protruding from a point, but when rotating, it appears circular in shape. Similarly, the individual rotating H2S molecules created the illusion of a closely packed crystal structure. However, at lower temperatures, the thermal motion of the molecule reduces. Hence, the true crystalline structure of the H2S can be determined, which is similar to that of ice.
This meant that H2S too can form hydrogen bonds. Later research has shown that amino acids like cysteine and methionine, which contain a sulphur atom, also par ticipate in hydrogen bonding.
The vague definition of a hydrogen bond involving only N, O and F has now become obsolete
The vague definition of a hydrogen bond involving only N, O and F has now become obsolete. In 2011, the official definition for hydrogen bond was revamped by the IUPAC – International Union of Pure and Applied Chemistry – the world authority on chemical nomenclature.
It now says: “The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation.”
Changing this definition was not an easy feat. It took a committee of 14 experts and 25 reviewers to reach an agreement. Some textbooks, however, have still not caught up with the new definition.
Searching for bonds
Back in 1960, when US chemists George Pimentel and Aubrey McClellan wrote the first textbook on the hydrogen bond, they suggested that a hydrogen bond can exist wherever there is evidence that a chemical bond specifically involves a hydrogen atom already bonded to another atom.
What does ‘evidence of a bond’ mean? How are these bonds visualised?
Usually, parameters like distance and angle between atoms are used to classify bonds. An almost straight angle (180 degrees) of the X–H – – Y (X and Y are the electronegative atoms) and the distance between Y and H being less than the sum of their van der Waals radii (a measure of atomic size) indicate the presence of a hydrogen bond.
One of the most commonly used techniques is Atomic Force Microscopy, which has provided beautiful pictures of the hydrogen bond. NMR and X-ray diffraction can also be used to distinguish normal and hydrogen bonds. NMR is especially useful in differentiating inter- and intra-molecular hydrogen bonds. Each peak in the NMR corresponds to the chemically inequivalent proton (hydrogen atom) of the molecule.
There are a number of NMR experiments available to establish the presence of intra- and inter-molecular hydrogen bonds, such as solvent dilution and temperature perturbation. These experiments reveal a significant displacement in the chemical shift positions of the protons par ticipating in the hydrogen bond. An interesting observation made by Suryaprakash’s group is the presence of hydrogen bond-mediated couplings of significantly larger strengths among the spins involved in the bond. Variations of the NMR technique have unambiguously established the presence of the hydrogen bond in many synthesised molecules.
Over the years, scientists have realised that the hydrogen bond may not be the only interaction of its kind
Over the years, scientists have realised that the hydrogen bond may not be the only interaction of its kind. One of the newer discoveries is the existence of a carbon bond between molecules. Similar to hydrogen, a carbon atom, when bonded to an element or group that can attract electrons, becomes par tially positively charged, and can interact with other electronegative atoms. Since carbon and its compounds form the basis of life, understanding these previously unexplored interactions can help us learn their roles in biological systems.
Halogen bonding, a new type of interaction, was also defined by an IUPAC task group in 2013. Similar to hydrogen bonding, this interaction depends on the development of a partial positive charge on a halogen atom – like chlorine or bromine – allowing it to interact with an electron-rich region in the same or different molecule.
The saga of the hydrogen bond shows that not only do many such intermolecular interactions exist that are yet to be discovered, but also the fact that the definition of a chemical bond keeps changing.
For example, Himansu’s group has been studying how hydrogen bonds are formed by sulphur and selenium, which are similar to oxygen. “We have recently predicted that metal hydrides can form hydrogen bonds,” he says. But this doesn’t fit the current definition of the hydrogen bond, because in metal hydrides, the metal atom is usually not more electronegative than hydrogen for it to attain a partial positive charge. “We are currently working on experimentally verifying this.”
Such findings, Himansu adds, suggest that we need a more “nuanced view” of what constitutes a hydrogen bond and the range of atoms that can participate in it. “The evolving definition of the hydrogen bond is a testament to Huggins’ initial, seemingly outlandish idea, which sparked a line of inquiry that continues to reveal the intricate ways in which atoms interact.”
Chandana Valaboju is a second year BSc (Research) student at IISc, and a science writing intern at the Office of Communications
(Edited by Ranjini Raghunath)




