With a mushrooming elderly population, research on ageing is gathering steam

Among Greek myths, the tale of Tithonus is particularly tragic. Eos, the immortal goddess of dawn, fell in love with the mortal Tithonus, a prince of Troy. She begged Zeus, the ruler of Greek gods, to grant Tithonus eternal life, but forgot to ask for eternal youth. Tithonus grew older but did not die; over time, his body withered away, leaving him babbling, broken and bitter. He eventually shrank down into a cicada whose incessant cries are supposedly heard even today.
A century ago, people were lucky if they lived past the age of 50. Today, a large number of elderly people face a fate similar to Tithonus’ – living longer but suffering from disease or poor health for decades.
“We have stopped falling off mountains in search of food. Wild animals are not killing us. Infectious diseases are more or less in control. But we are getting older,” says Deepak Saini, Professor at the Department of Developmental Biology and Genetics (DBG), IISc. “Ask an audience how many want to live long, most will shout yes. Ask how many want to be old, perhaps none will answer.”
With nearly 1.43 billion people, India’s population just crossed China’s to become the largest in the world. By 2050, every fifth Indian will be over 60 years of age, according to a UN Population Fund report. Chronic diseases like cancer, diabetes and Alzheimer’s are on the rise among the elderly. “People usually retire by the age of 60-65, and most of them can live up to about 85. There’s a very real pressure to identify what can be done to keep them healthy during those 20 years,” explains Deepak. The idea of boosting one’s “healthspan” – how long an individual lives in general good health – is now taking centre stage in debates raging around ageing.
But before we get to healthy ageing, we need to understand the process of ageing itself. When does ageing actually begin in the human body? Is ageing accelerated by genetic or environmental factors? Why do women live longer than men? Can we really reverse ageing? Scientists are only now beginning to find the answers to some of these questions. “We know so much and yet so little,” says Deepak.
Why do we age?
In the 19th century, some people believed in a weird theory about why we age: Our bodies have a limited supply of “vital energy” assigned at birth that gradually depletes over time. The key to delaying ageing, they recommended, was to save as much energy as possible by refraining from “excesses and undue exertions”, eating specific foods and practising abstinence.
While this theory has long been debunked, over the years, scientists have put forth dozens of more plausible explanations for why our bodies age.
Some scientists believe that our lifespan is set at birth and ageing unfolds in a “programmed” manner, controlled by genes turning on and off at specific times, or by the release of certain hormones.
The “wear and tear” theory suggests that just like a car, our body parts become rusty over time, mainly due to continuous assault by free radicals (unstable molecules that build up as a result of chemical reactions in the cell). A related theory suggests that a faster metabolism might be linked to quicker ageing. “The idea is that more free radicals are generated when burning more fuel. Therefore, the more the system’s going to get injured,” says Deepak. This might explain why excess exercise has sometimes been linked to faster ageing – a 2022 Harvard study of about 3,000 former football players found that their healthspans were shorter than the general population by nearly a decade, and they also appeared to develop arthritis and dementia earlier. “Exercise seems to keep you healthy but may not correlate with you living longer,” Deepak adds.
One theory is that ageing might be a defence mechanism against cancer
The theory that most scientists are invested in, however, is that ageing might actually be a defence mechanism against cancer. Ageing cells have a curious property called senescence, triggered by stresses of different types. When we are young, our cells are able to repair injuries rapidly and cells beyond repair are quickly cleared out by the immune system. As we grow old, the immune system and the cell’s repair machinery become weak, but stresses and assaults continue, triggering inflammation all over the body, creating a ripe environment for cells to turn cancerous. To avoid this, damaged cells switch to a limbo-like state called senescence in which they stop dividing – which is critical for cancers to form and spread – but continue to eat food, remain active and talk to other cells.
“The damaged cell neither wants to die nor does it want to become cancerous. It is in a finely tuned state where the cell just maintains its vital status without proliferating,” explains Deepak.
But senescence can be a double-edged sword. As the number of senescent cells grows in the body, they start affecting other healthy cells, putting strain on the body’s resources and reducing our ability to fight off infections. “Slowly, the equilibrium starts shifting towards a greater number of aged cells, and a lesser number of cells which can clear them out. Once this balance tips over, you enter into what is called physiological ageing,” explains Deepak.
Ageing and the human body
When I started getting my first grey hairs at the age of 30, I was instantly jealous of my grandfather whose hair remained dark well into his sixties. Not all ageing individuals look alike, but there are some signs, like greying hair, that are more or less universal. Our skin starts to wrinkle. Our senses become weaker. Spicy foods suddenly become intolerable. Blood vessels become stiffer, making it harder for our hearts to pump blood. Bones become brittle and break easily. A weaker bladder makes us run more frequently to the restroom.
With age, our muscles also start losing mass and flexibility. Upendra Nongthomba, Chair and Professor at DBG, and his lab use fruit flies to study how muscle function breaks down over time. They have specifically been investigating the crosstalk between muscles and neurons at sites called neuromuscular junctions. Their experiments showed that flies with mutations that disrupt this crosstalk seem to age faster.
Upendra’s lab has also been focusing on the build-up of protein aggregates in muscles with age. “Our muscle proteins are cleared out and renewed every few days. As you age, this clearance machinery will become faulty, so you will have increased aggregation.” His lab is working with P Thilagar, Professor in the Department of Inorganic and Physical Chemistry (IPC), to develop artificial molecules that can break up these aggregates.

Another major change inside the bodies of older people is an increase in inflammation. Inflammation is the immune system’s immediate response to injury or infection, which lasts up until the injury heals or the infection is treated. However, in older people, inflammation becomes chronic – it is constantly present at low levels and further exacerbates ageing, a condition called “inflammaging”. Excess inflammation is dangerous and can greatly damage cells, but as people age, their ability to clamp down on inflammation becomes weaker. Deepak’s lab has uncovered the role of a critical cell surface protein called CXCR4 involved in driving this inflammation. Targeting this receptor can potentially help control inflammation and allow ageing to happen at a normal rate, he explains.
But a high level of inflammation also seems to have a curious and counterintuitive benefit: It can help older people fend off infections. In an aged body, the immune system is already weak, and unable to quickly identify infected cells. “Inflammation is like an innate defence mechanism. Higher inflammation by itself can counteract infection,” says Deepak. “We showed [in mice models] that in the case of at least two intracellular pathogens – Salmonella and Mycobacterium – the infection burden in aged animals is lower and it is mediated by enhanced nitrosative stress [overproduction of nitrogen-based free radicals] which goes up in ageing.”
A high level of inflammation can help older people fend off infections
This may also explain why giving drugs that suppress inflammation to older people puts them at a higher risk of infections, according to Deepak. During COVID-19, for example, many doctors were probably not looking closely enough at what drugs older people were already taking, he says. “If they were co-morbid, they were definitely taking some drugs which might have suppressed their inflammatory response. This is one part we are actively exploring – to understand if the aged host’s susceptibility [to infection] is altered because of the drugs we give them. That means we need to revisit therapy for the aged.”
Ageing in the brain
Even in his seventies, my grandfather could reel off names of classmates and teachers from his middle school days. But not all older people are that lucky.
People who develop dementia start losing their memory early on, becoming more and more forgetful with time, often not recognising their own family members. They start asking the same questions repeatedly, become confused and disoriented, and sometimes wander off on their own.
It is to study such neurodegenerative disorders that the Centre for Brain Research (CBR) was established on the IISc campus in 2015 with a philanthropic grant of Rs 225 crore from the founders of the Pratiksha Trust, Kris and Sudha Gopalakrishnan. Earlier this year, the Trust made a fresh commitment of Rs 450.27 crore spread over 10 years.
A large part of the research at the Centre has focused on understanding how the brain ages in a healthy individual. Under two long-term studies planned out over 10-15 years, one focusing on about 10,000 individuals living in the Srinivasapura Taluk (about 100 km from Bangalore) and the second on about 1,000 individuals in Bangalore city, researchers have been tracking how genetic, environmental and socio-economic differences shape brain ageing.
A large part of the research at the Centre for Brain Research has focused on understanding how the brain ages in a healthy individual
Once a year, people recruited for the two studies are tested for dozens of biomarkers in their blood, get their genomes sequenced and undergo brain imaging scans. The data are then pooled together to build a comprehensive picture of how their brain health is changing every year. One of the reasons why Srinivasapura was chosen is because it is home to many mango orchard farmers who have lived in the area for generations with very few migrating away, making it easier to follow up with participants for years.
“We want to see the progression of ageing, and how multiple factors including comorbidities contribute to either healthy ageing or cognitive difficulty,” says Ravi Muddashetty, Associate Professor at CBR. “This is a unique project in the Indian context and a rare one even globally.” He adds that researchers at CBR have already collected a wealth of data from these studies and are currently analysing them.
Apart from these large-scale studies, researchers like Ravi are also zooming in on cellular and molecular-level changes in the ageing brain. His lab is particularly tracking the connections between neurons that form and break down over time. When we are born, our brain has about 100 billion neurons, the most we will ever have in our lifetime. Right after birth, these neurons get busy forming connections with neurons in other parts of the brain and the body. This continues up until the age of 25-30. “By 30, you get what you can call an optimally functioning brain,” explains Ravi. “From then onwards, every year, there may be some reduction in the number of connections the neurons make. We are studying what factors determine making and breaking or stabilising these connections.”
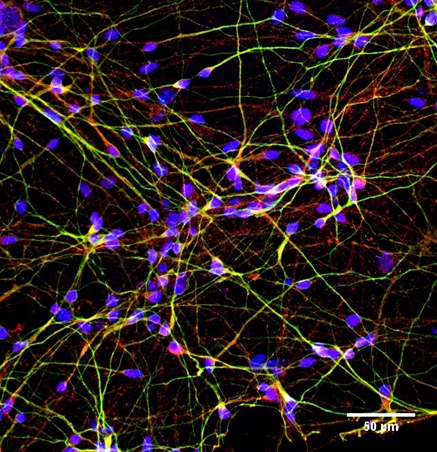
Alzheimer’s patient (Image: Sarayu Ramakrishna)
As these connections deteriorate with age, brain functions like memory, learning and cognition slow down. Other changes also add up: Some parts of the brain start shrinking, blood supply gets reduced, and most importantly, aggregates of proteins begin building up in the brain – the hallmark of many neurodegenerative diseases like Alzheimer’s and Parkinson’s.
“Neurons are non-dividing cells. In a dividing cell, any malfunctioning proteins can be gotten rid of. But in the case of neurons, you can’t have that function and there is a slow accumulation of mistakes,” says Ravi. “This accumulation of misfolded proteins or aggregates leads to unhealthy ageing and diseases of cognition.”
There is also growing evidence that brain health may be influenced heavily by the trillions of tiny microbes that live inside our intestines – the gut microbiome.
“The gut-to-brain axis is one of the new areas being studied extensively,” Ravi says. “What we eat and the way we live determines our gut microbiome, which in turn has an influence on ageing.”
Flies, worms and food
Studying ageing in humans is not easy because changes happen over decades. This is why scientists have turned to smaller animals like fruit flies and worms that have much shorter lifespans. “Usually, fruit flies live for close to 65-70 days. But we’ve developed mutant flies that can live up to 150 days, and also those with only half the lifespan, around 30-35 days,” says Upendra. His team is able to track the growth of an entire organism from birth to death within a few weeks, and study how ageing unfolds.
Another model organism that has proved useful in ageing research is C. elegans, a tiny transparent worm that has a life span of two to three weeks. Many proteins in the worm have functions very similar to human proteins. “Its tissue structures are also similar,” explains Varsha Singh, Associate Professor at DBG.
These flies and worms are helping Varsha and Upendra uncover exciting insights into the links between food, gut and ageing.
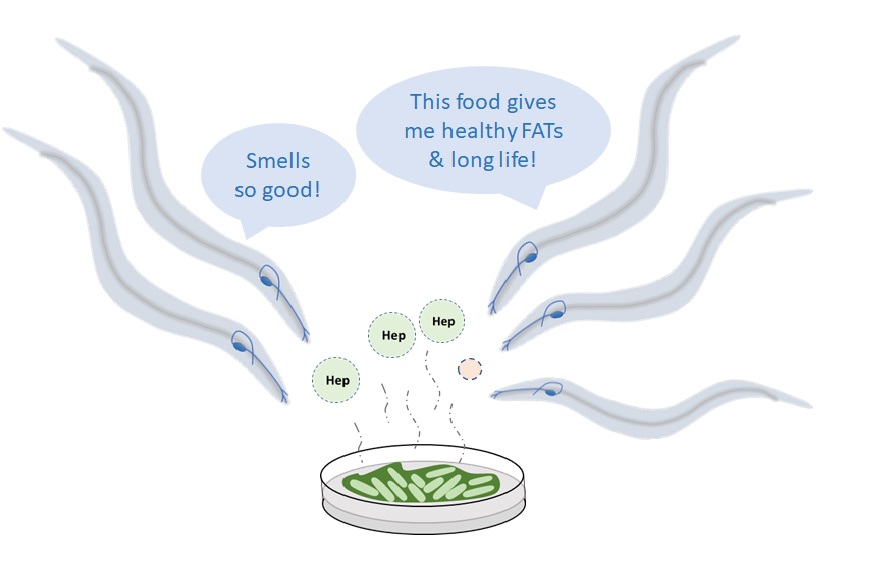
When Varsha began studying ageing in worms, a key question she was interested in was how the animal smells specific odours. To live longer and “produce more babies”, the worm first needs to find the right food, and to find the right food, it needs to have a good sense of smell. “We started with a simple question: If we alter olfaction [the ability to smell] in C. elegans, do we see changes in how long the organism lives?” In their experiments, her team found that long-lived worms have a specific protein in their neurons that can “smell” a compound called 2-heptanone, emitted by bacteria that the worm needs to eat. The same smell is produced by some bacteria in our gut, like the ones that we take in with curd. The receptor protein for the smell is responsible for regulating lipid metabolism, which in turn is important for regulating lifespan in worms. Varsha and other researchers suggest that this protein is able to sense 2-heptanone released by microbes in our gut, and this sensing can therefore boost our health too.
In addition, the same study by her team showed that short-lived worms lacked mono unsaturated fatty acids (MUFAs), the kind of fats found in foods like almonds. When these worms’ diet was supplemented with MUFAs, they lived longer.
Such findings emphasise how the food we eat can influence health and lifespan. “For example, it is believed that in certain Scandinavian countries, people are longer-lived because they have lots of cabbage in their diet,” explains Varsha. “The idea is that cabbage changes the gut microbiome, which produces something that will improve health.”
Varsha and Upendra believe that some dietary supplements, including Ayurvedic and traditional medicine, might help boost health during ageing. Upendra has been testing some of these formulations in his lab. His team has found that a specific molecule present in pomegranate juice could help fruit flies remain healthy for longer. His experiments have shown that some salts and salt solutions described in Ayurvedic medicine can reduce gut leakiness in ageing flies by modulating gut bacteria. Upendra hopes that identifying and scientifically validating some of these compounds could lead to therapies that can counter or delay the symptoms of ageing. He adds that the department is also trying to set up a full-fledged centre that will not only focus on understanding the biology of ageing, but also develop therapies that can promote healthy ageing.
In recent years, many therapies have been proposed that claim to stop or reverse ageing. Some researchers have also been testing existing medicines like metformin (an antidiabetic medication) and rapamycin (an immunosuppresive drug) for their ability to counteract ageing-related complications. Countries like Saudi Arabia are reportedly spending a billion USD annually to develop treatments that can slow down ageing.
So far, however, anti-ageing therapy has remained a pipe dream. “People have found some [initial] success with a lot of molecules. Unfortunately, most of them have failed in controlled trials,” says Deepak.
“The only thing which works without any doubt is caloric restriction. It is the only known therapy which is known to extend lifespan across lifeforms – from worms to flies to mice to humans,” he adds. “If we eat less, we burn less, and we tend to live longer.”




