
P N Rangarajan is currently the Chair of the Department of Biochemistry, IISc. He obtained his PhD from IISc in 1989 and carried out his postdoctoral work at the Salk Institute for Biological Studies, USA. Since joining IISc as an Assistant Professor in 1993, he has carried out research in the field of eukaryotic gene expression and vaccine development. Besides being an elected fellow of three major Indian science academies, he was also awarded the Shanti Swarup Bhatnagar Prize in 2007 in recognition of his outstanding scientific contributions.
During the late 1990s, Rangarajan developed an indigenous recombinant Hepatitis B vaccine which was subsequently marketed by multiple companies. This vaccine has been a huge commercial success, with hundreds of millions of doses sold so far. Rangarajan spoke to Connect about how the vaccine was developed, how it was taken to the market, and the impact that it has had. Here are excerpts from the interview.
Why did you decide to do research on Hepatitis B?
I joined the Department of Biochemistry in 1993 as an Assistant Professor. Like everybody else, initially, I also focused on extending my postdoctoral work which was on transcriptional regulation by retinoic acid receptors. But in the back of my mind, I always wanted to do work that has some benefit for the country. I was greatly influenced by my PhD mentor, Prof G Padmanaban, who played a key role in the establishment of the Astra Research Centre in Bangalore, and by my postdoc mentor Prof Ron Evans at the Salk Institute, San Diego, who had a tie-up with a company called Ligand Pharmaceuticals through which his research was translated to developing drugs. I always thought that, in addition to my basic research, I should also do something that is of relevance to the country.
Such an opportunity came in 1998, when a company called Bharat Biotech approached me for developing the indigenous recombinant Hepatitis B vaccine. In the 1990s, India was importing this vaccine from abroad for immunisation. However, because it was expensive, the government was unable to purchase large volumes of this vaccine required for the Universal Immunisation Programme. Additionally, in 1996-98, the Ministry of Human Resource Development introduced a scheme called Technology Development Mission (TDM) under which scientists were encouraged to collaborate with industry to develop indigenous products that are useful for the country.
Even though I had no prior experience in working with yeast, I took up this challenge and collaborated with Bharat Biotech to develop the recombinant Hepatitis B vaccine under the TM.
How did you develop this vaccine in your lab and how does it work?
As I mentioned earlier, in the late 1990s, India was importing this vaccine and the major market share was for a brand called Engerix B which was made from Saccharomyces cerevisiae or Baker’s yeast. The technology for the vaccine was already available, but what we did was indigenisation of this technology.
We decided to use a different type of yeast called Pichia pastoris which can grow to higher cell densities compared to S. cerevisiae and can therefore produce greater amounts of the recombinant protein. We cloned the gene encoding the Hepatitis B virus surface antigen (HBsAg) and expressed it in P. pastoris. Recombinant P. pastoris strains expressing high levels of HBsAg were selected. We also standardised a four-step protocol for purifying HBsAg from the yeast cell extracts. This purified HBsAg, when adsorbed onto aluminium hydroxide and injected into humans, generates anti-HBsAg antibodies in the body. These antibodies bind to the virus and prevent it from infecting liver cells, thereby conferring protection. This was the recombinant Hepatitis B vaccine that we developed.
The technology for the vaccine was already available, but what we did was indigenisation of this technology
Before we could complete the project, a company called Shantha Biotech introduced a P. pastoris-based Hepatitis B vaccine (Shanvac-B) in the market. However, since this vaccine had a huge market, several companies wanted to manufacture the vaccine in India using indigenous technology. Therefore, I transferred the technology to three companies in Hyderabad, of which two acknowledged our contribution.
How did this vaccine reach the market from the lab?
After the vaccine was developed, the technology was transferred to Bharat Biotech and the company launched the product. Unfortunately, it did not acknowledge IISc’s contribution. After this misadventure, two other companies in Hyderabad, Biological E Limited and Indian Immunologicals Limited, approached me because they knew that I had a commercially viable technology.
After getting the recombinant P. pastoris yeast strain from us, both these companies carried out the necessary clinical trials, obtained the regulatory approvals, and launched the vaccine under the names BEVAC and Elovac-B, in 2004 and 2006, respectively. Both these vaccines are called monovalent vaccines since they have only one component: the Hepatitis B virus surface antigen. When this monovalent vaccine is combined with four other vaccines – Diphtheria, Pertussis, Tetanus (DPT), and Haemophilus Influenzae B (HiB) – you get a pentavalent vaccine. In 2011, Biological E Limited brought out a pentavalent vaccine called ComBE Five, and in 2018, Indian Immunologicals Limited brought out their pentavalent vaccine called Vaxtar-5.
Both the companies not only brought out these vaccines and duly acknowledged IISc’s contribution in the vaccine development programme, they also paid one percent royalty on total sales and fully honoured the terms and conditions of the MoU we signed with them. In fact, after the successful completion of this project, Indian Immunologicals Limited signed another MoU with my lab via the Society for Innovation and Development (SID), IISc, to develop a DNA rabies vaccine, since they wanted me to support their company’s R&D. They actually gave me Rs 50 lakh per year as an R&D grant for five years. During the next five years, we had a very good collaboration and I helped them establish a recombinant DNA laboratory.
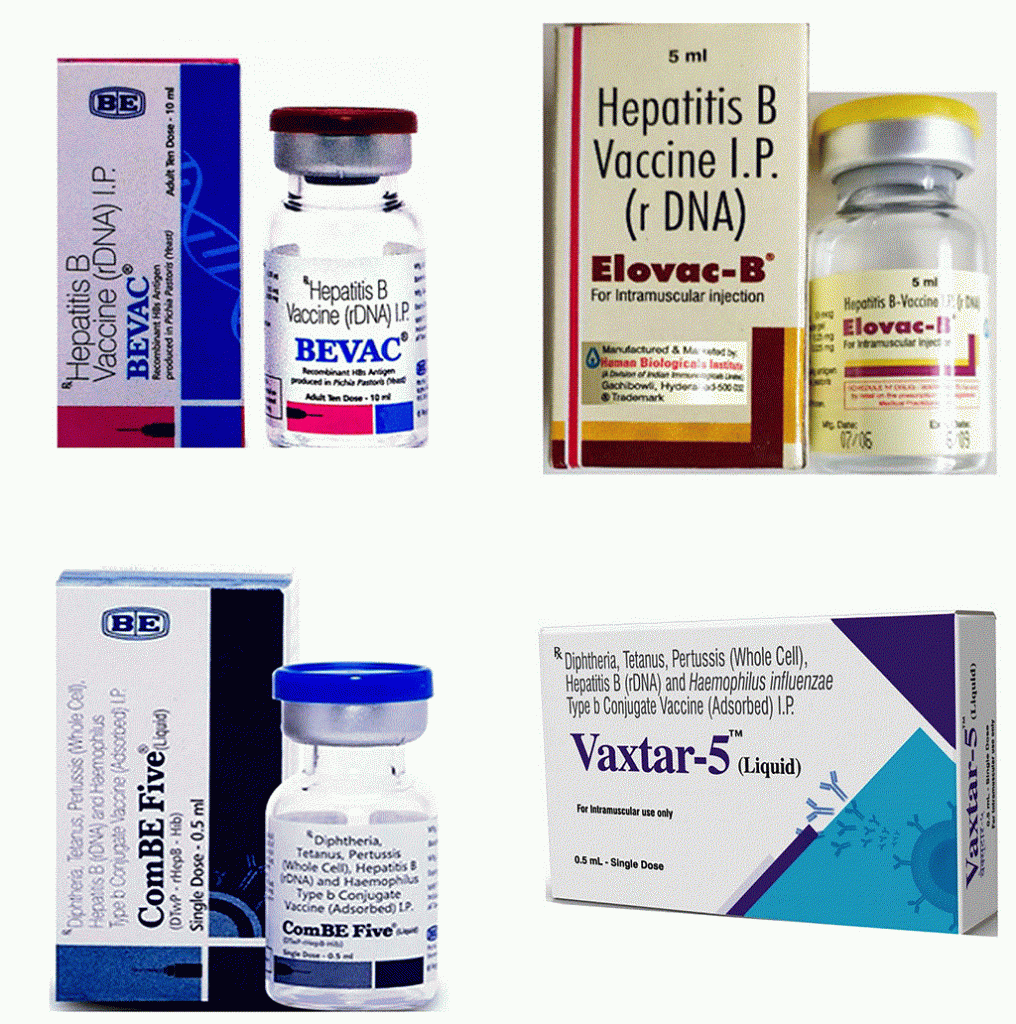
provided by Rangarajan (clockwise from top-left): BEVAC, Elovac-B,
Vaxtar-5, and ComBE Five (Photo courtesy: PN Rangarajan)
What has been the impact of these vaccines in the market?
The indigenous manufacture of these vaccines by several companies in India led to a drastic reduction in their prices, enabling the Government of India to announce their inclusion in the universal programme of immunisation. In 2016, Biological E Limited obtained an order of Rs 895 crore, while in 2018, Indian Immunologicals Limited obtained an order of Rs 210 crore from the Government of India to supply their pentavalent vaccines for the Universal Immunisation Programme.
When I asked these companies whether they are still using the yeast strain provided by me, they both told me in writing that yes, both of them are still using the Pichia strain received from IISc for the production of their Hepatitis B vaccines. Biological E Limited has sold more than 110 million doses of the BEVAC brand since 2010-11 and more than 200 million doses of the pentavalent vaccine since 2012-13. Indian Immunologicals Limited has sold more than 110 million doses of the Elovac-B brand since 2006 and more than 3 million doses of the pentavalent vaccine (Vaxtar-5) since 2018.
It is very gratifying that a technology that I transferred in 2002 is still being used for making millions of doses of vaccines. Millions of children are being immunized against Hepatitis B with a vaccine made from a yeast strain developed in my lab. This vaccine is an example of making a global technology work locally, resulting in the reduction of the vaccine’s cost and its introduction in the Universal Immunisation Programme. We, at IISc, have made a small contribution towards this effort.
Personally, what were the major challenges you faced during the development of this vaccine?
In the 1990s, very few faculty members in the Division of Biological Sciences at IISc were engaged in collaboration with industry. The mandate was to carry out basic research, publish high-quality papers, and train graduate students. I was probably the first Assistant Professor in the Division who collaborated with the industry before being promoted to Associate Professor. I chose to travel down the road not taken. It was quite disappointing when IISc refused my promotion to an Associate Professor at that time, since, in my excitement to develop the vaccine, I had not published enough papers.
It is very gratifying that a technology that I transferred in 2002 is still being used for making millions of doses of vaccines
The second challenge was when Bharat Biotech, the first company to get the technology from us, did not acknowledge our contribution. I had to spend a lot of time convincing the IISc administration that we should give this technology to other companies because of its national importance. I am grateful to Prof G Padmanaban, the then-DBT secretary Dr Manju Sharma, Prof M Vijayan, who was the Associate Director at that time and also in-charge of TDM, and of course my family for their help, encouragement, and for supporting me in difficult times. Looking back, I did go through tough times. But in the long run, my decision to work with companies and develop an indigenous vaccine gained appreciation in the country.
In addition to the Hepatitis B vaccine, you have also worked on developing vaccines against rabies and Japanese encephalitis. Could you briefly tell us about those?
Along with the Hepatitis B vaccine project, I had also started a research programme on developing a DNA vaccine in which, instead of a protein, a plasmid DNA encoding a viral antigen is directly injected into the body. Unlike the recombinant Hepatitis B vaccine, which is an example of indigenisation of available technology, the DNA vaccines were very innovative products since nobody had launched a DNA vaccine for human use in the market. While we published good papers and even obtained a patent, unfortunately, we did not succeed in bringing out the Japanese encephalitis vaccine or rabies vaccine into the market.
Are there any more vaccines in the pipeline from work that is currently being carried out in your lab?
I have stopped the vaccine work right now as it was becoming very difficult for me to do both basic as well as applied research. When I was working on Pichia pastoris, I realised that it is a wonderful yeast because many aspects of its biochemistry and metabolic pathways are very different from Saccharomyces cerevisiae, the yeast on which most of the research work was being done. I thought that this is a goldmine for basic research.
Therefore, my entire lab is now focusing on understanding the regulation of metabolism of P. pastoris. We have identified several transcription factors and novel regulatory mechanisms and have had good publications in thenpast 10 years. We are now beginning to see some interesting leads which have the potential to contribute to a novel expression system.
What do you see as the major challenge in the field of vaccine development?
India has become a major player in the global vaccine market through the indigenous manufacture of vaccines. But, to the best of my knowledge, we are yet to bring out an innovative, blockbuster vaccine into the global market through our own R&D efforts. This, in my opinion, has not really happened and is the major challenge. For example, the rabies vaccine that I had developed was a home-grown technology for which we got a patent, but somehow it could not be converted into a commercially successful product. Now, there is a lot of encouragement and a number of programmes to support academia-industry interactions, biotech startups, and entrepreneurs. It is up to the faculty members to take up this challenge and develop commercially successful products.
Madhura Amdekar is a former Research Associate at the Centre for Ecological Sciences, IISc.




