American neuroscientist Michael Zigmond’s research shows that physical exercise could go a long way in fighting Parkinson’s disease

Michael Zigmond is a believer. “I don’t know why anybody wouldn’t want to be a neuroscientist,” quips the IISc-DST Centenary Chair Professor. Notwithstanding his evangelism about the virtues of a career in neuroscience, Zigmond found his own true calling by accident. In the early 1960s, when he was studying to become an engineer at the Carnegie Mellon University (then the Carnegie Institute of Technology), he was told that he was required to take a course in psychology in his third year. “I tried to get out of it. I told my Chairman that I got along pretty well with people,” he recalls. When his plea failed, Zigmond grudgingly signed up for the course. But by the end of the course, which he says was taught from a biological perspective, he was more excited by chemical transmission between neurons than chemical engineering, his major. So he switched to neuroscience for his PhD. Now 76, his passion to understand the mysteries of the brain remains undiminished.
Neurodegenerative Diseases
Zigmond is an emeritus professor in the Department of Neurology at the University of Pittsburgh, where he has worked since 1970. He has spent decades studying Parkinson’s disease (PD), considered a progressive neurodegenerative disease, progressive because the condition worsens with time and neurodegenerative because it involves the loss of neurons in the brain.
Though they are typically associated with old age, the origin of the various neurodegenerative diseases and how they are related to each other is still not entirely clear. “I don’t think we have a good understanding of this yet, but let me try to answer it with something most people haven’t heard of: the Guam complex,” Zigmond says.
For centuries, seeds of a cycad plant were used by a group of people in the Pacific island of Guam as part of their diet and as a source of medicine. But the seeds had to be washed repeatedly and prepared with great care because they have a neurotoxin in them. However, by the 20th century, people had stopped consuming these seeds as they switched to a western diet (it is a US territory). When World War II started, there was a shortage of food on the island. “So they had to make their own food. They knew that you could make flour from the cycad plant but didn’t know how to clean it,” Zigmond says. Not long after, the incidence of neurodegenerative diseases among these islanders rose dramatically, an increase that has been attributed to the consumption of the cycad seeds. What makes this even more interesting is that not all of them got the same disease. “Some people got Parkinson’s, some people got Alzheimer’s, some people got ALS [Amyotrophic Lateral Sclerosis],” adds Zigmond to illustrate the complex interaction between genetic factors and environmental triggers that give rise to this suite of neurological disorders.
While neuroscientists are yet to unravel this enigma – among many others – they have over the years learnt quite a bit about neurodegenerative diseases, including PD, which Zigmond specialises in.
Parkinson’s Disease
Most of the visible symptoms of PD – tremors, loss of balance, slowed walking, and muscle stiffness – are related to movement. But they are physical manifestations of a deeper neurological malaise: the degeneration of dopamine neurons.
Most of the visible symptoms of Parkinson’s are related to movement. But they are physical manifestations of a deeper neurological malaise: the degeneration of dopamine neurons
Dopamine is a chemical messenger implicated in several cognitive processes and emotional states including love, addiction, motivation, and attention. It is also crucial for normal motor functioning. Many of the dopamine neurons sit in a narrow passage in the brain (the passage connects the substantia nigra in the lower brain to the striatum in the basal ganglia of the upper portions of the brain). When these neurons die, it results in a loss of dopamine to the brain, thus affecting normal movement of the body.
“So what’s the evidence that Parkinson’s disease is caused by loss of dopamine?” Zigmond asks. But before he answers this question, he takes a detour to narrate the compelling history of neuropharmacology, one which began in India.
Indian Roots of Neuropharmacology
“For at least 4000 years,” Zigmond explains, “people in India have used the root of this flowering shrub called Rauwolfia serpentina [known in Sanskrit as sarpagandha] to make a tea to treat a wide variety of conditions which seem to be unrelated: hypertension, anxiety and insanity, which we would now call schizophrenia.”
The 1930s and 1940s saw a surge in the popularity of sarpagandha as a medicine when several pharmacological experiments were conducted on its efficacy in India. Among the more influential studies was Rustom Jal Vakil’s demonstration of its utility in treating hypertension (Mahatma Gandhi was known to use it regularly as a tranquiliser). Its reputation soon spread to Europe and North America. There was excitement in the global scientific establishment as well with over a hundred papers published on Rauwolfia in less than five years.

Reserpine
Among the major alkaloids found in Rauwolfia, the most significant – at least for humans – is reserpine, first isolated in 1950. “This molecule is what is responsible for all these amazing things. Its discovery kick-started the field of neuropharmocology,” Zigmond says. And it was soon marketed as a drug called Serpasil by the Swiss pharma giant Ciba Labs.
Hailed as a wonder drug, reserpine was now being used to treat various neurological conditions. But it required another breakthrough about reserpine for researchers to understand how PD is associated with the loss of dopamine. And that came from Arvid Carlsson, a Swedish Nobel Laureate who died only this summer at the age of 96.
Dopamine and Parkinson’s Disease
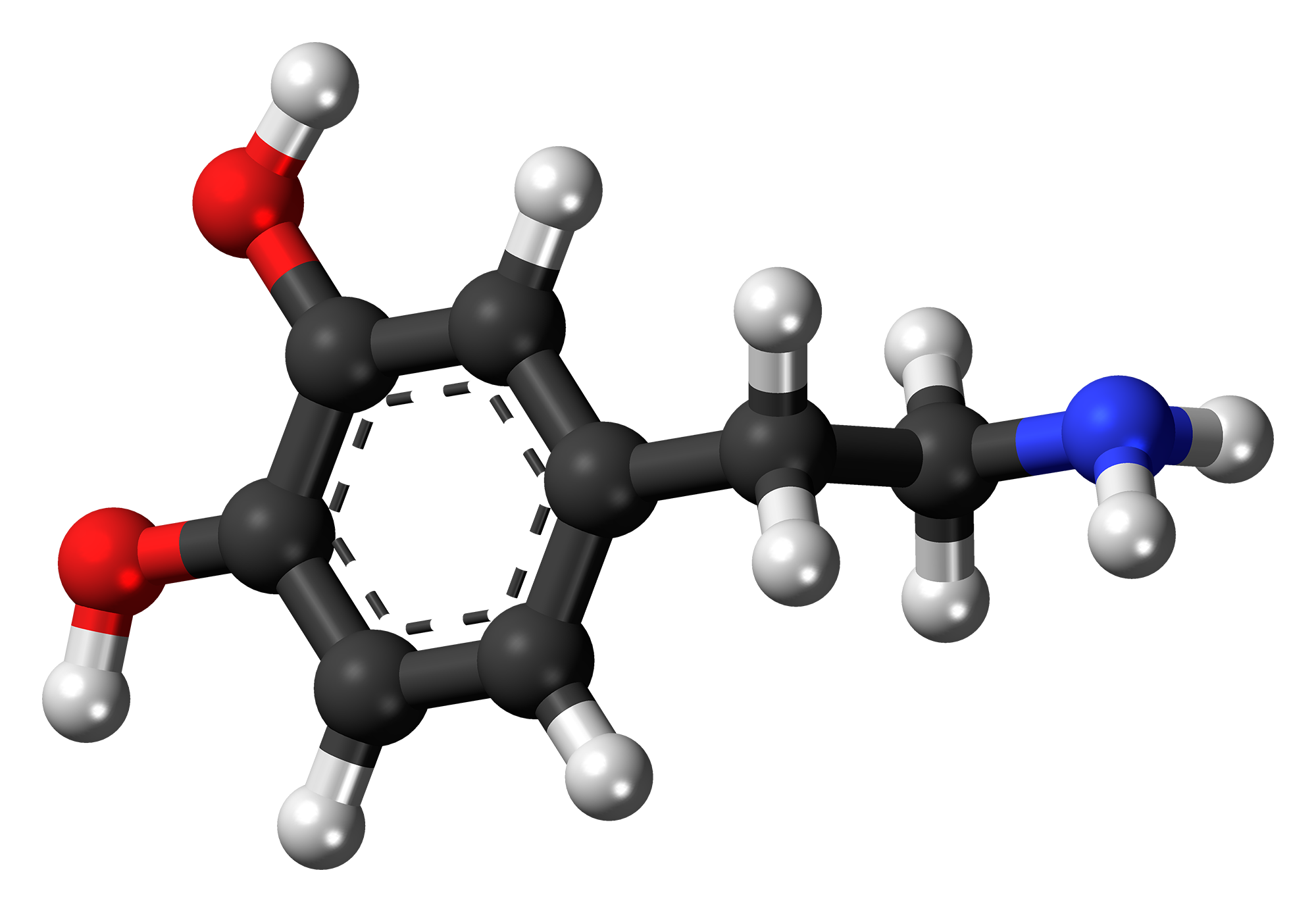
Back in the 1950s, according to Zigmond, Carlsson was among the many scientists enamoured by reserpine. In one of his experiments, he injected rabbits with reserpine. “They became completely flaccid, completely akinetic. They stayed that way for almost 24 hours.” Carlsonn hypothesised that reserpine’s effect on the rabbits was either due to serotonin or dopamine (dopamine’s presence in the brain had just been discovered that year but was yet to be established as a neurotransmitter). “So he injected the precursor to serotonin and the precursor to dopamine called L-dopa [into the akinetic rabbits],” Zigmond continues. Carlsonn found that L-dopa restored electrical signalling in the rabbit brain and reversed behavioural deficits, thus demonstrating unambiguously that dopamine is a neurotransmitter.
L-dopa restored electrical signalling in the rabbit brain and reversed behavioural deficits, demonstrating that dopamine is a neurotransmitter
Limitations of Current Treatment
But after the burst of frenetic discoveries in the late 1950s and early 1960s, little progress has been made in how we treat PD, claims Zigmond. “People still use L-dopa and a few other drugs like L-dopa to treat Parkinson’s. But they have no effect on the disease itself; it just masks the symptoms. And in fact, L-dopa becomes less effective with time.” (Dopamine itself is not used as a drug because it struggles to break the blood-brain barrier.)
Exercise
A few years ago, Tim Schallert, a researcher at the University of Texas at Austin, called Zigmond up (Schallert, who was a close friend of Zigmond, died earlier this year from complications due to PD). “He [Schallert] said that maybe exercise could protect against Parkinson’s. I said that’s crazy.” Zigmond clarifies that there had been some studies published on exercise and PD, but their focus was on how exercise could ameliorate the symptoms of PD. None of them had considered the possibility that regular physical activity could provide what Zigmond calls “neuroprotection”. In other words, these studies did not ask whether exercise could delay the progression of the disease, and perhaps even prevent it. And why would they, he thought. Exercise affects muscles. There was no reason he could think of to assume that it would have anything to do with the health of neurons.
“He [Timothy Schallert] said that maybe exercise could protect against Parkinson’s. I said that’s crazy.”
Schallert persisted. And prevailed in convincing his friend to do a joint study. As a first step to test their ambitious idea, the two researchers (along with their colleagues), injected a toxin to selectively kill dopamine neurons in one side of the brains of rats and induced motor neglect – the failure to move muscles voluntarily – in their right front limbs, mimicking the motor effects of PD. The animals were then put in cylindrical observation chambers. “What the rats will do is to rear up on their hind limbs and explore the chamber. They like to explore just like we do,” says Zigmond. But because of motor neglect, they would explore with their left limbs and also land on them.
They then put casts on the “normal” limbs of the rats compelling them to use their impaired limbs (Zigmond credits Schallert for coming up with this enterprising trick). After seven days of forced exercise, they removed the casts and observed the rodents. What they found surprised them: the rats were now using both their limbs with equal proficiency.
Neuroplasticity
There was more good news. In the same paper, the authors also demonstrated that exercise more than just improves motor functioning; it also alters the underlying neurochemistry. This experiment was led by Ann Cohen, an assistant professor at the University of Pittsburgh, who was then a graduate student in the Zigmond lab. She showed that the depleted dopamine in the neurons of the substantia nigra made a comeback after merely seven days of exercise. “So it seemed like it wasn’t just behaviour that is protected, neurons are also protected,” Zigmond asserts.
Schallert, Zigmond and their colleagues demonstrated that exercise more than just improves motor functioning; it also alters the underlying neurochemistry
Zigmond and his collaborators followed up this finding with more studies. They used mice and monkeys. They changed the way the animals exercised – with hamster wheels and treadmills. They increased the duration of exercise. The results of these experiments only confirmed that regular physical exercise provides neuroprotection, at least in animal models.
Mechanisms of Neuroprotection
A huge body of literature has demonstrated how moderate physical activity alters human physiology at the cellular level: it increases mitochondrial ATP synthesis and antioxidant defences and reduces inflammation and ROS (Reactive Oxygen Species). Now we know that it can also increase the number of neurons and synapses in the brain. Remarkably, these are exactly the opposite effects of PD. “So exercise seems to reverse many of these factors that we know are altered in people with Parkinson’s disease,” Zigmond says.
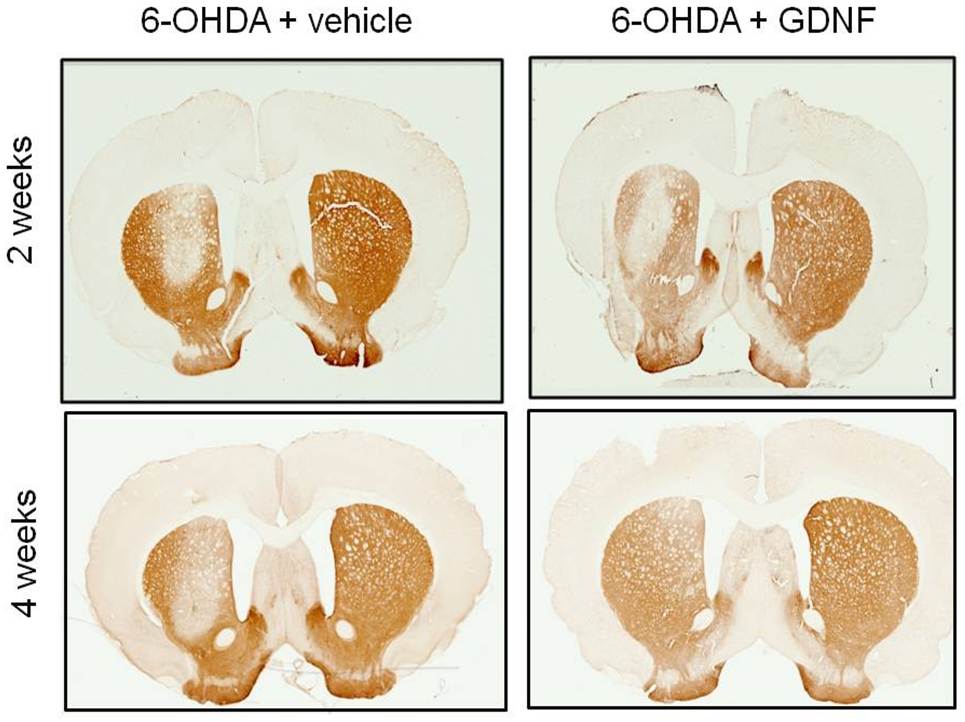
But Zigmond is most excited by one other physiological change that exercise brings about: an increase in neurotrophic factors, biomolecules known to support the development, growth and survival of neurons. He was particularly interested in a neurotrophic factor called the Glial cell line-derived neurotrophic factor (GDNF) because it had also been shown to be neuroprotective by several researchers, including Barry Hoffer, then at the National Institutes of Health.
“We know that exercise increases GDNF; we know that GDNF protects dopamine neurons. So our hypothesis was very simple: an increase in GDNF is the primary mechanism through which exercise provides neuroprotection,” Zigmond explains.
“Then Annie [Ann Cohen] said, ‘If this hypothesis is correct, we can bypass exercise. We can put GDNF directly into the brain, and that should be just as good as exercise,’” recalls Zigmond. So Cohen injected rats with both the dopamine-killing toxin and GDNF. But there was no change in the number of dopamine neurons in the first couple of weeks. “I was with Annie when she collected this data. She was heartbroken because this was going to be her PhD thesis. But miraculously in two months the “degenerated” dopamine neurons came back.” Apparently, they had just turned off their capacity to show the typical markers of dopamine while they fought off the effects of the toxin.
Following this significant discovery, Zigmond and his team have also been able to understand the molecular underpinnings of how GDNF protects dopamine neurons. They did these experiments not on animals but on cellular models in petri dishes.
Environmental Enrichment
But it’s not just exercise which seems to trigger rejuvenation of the brain. “Richard Smeyne [also a collaborator of Zigmond’s, from Thomas Jefferson University] came up with the idea of taking mice and putting them in what he called an enriched environment,” Zigmond says. “Six to twelve mice were kept together for three months in a large living space containing exercise wheels, toys, and objects to climb over or under. And they kept changing things around every three to five days to keep the environment novel. The mice did what kids do in a playground – they explored, they played, they interacted,” he elaborates.
At the end of three months, when the researchers dissected the brains of these mice, they found that it had effects similar to what they observed with exercise, even though the amount of physical activity they indulged in was much less. They found more neurons and synapses, and lower levels of inflammation and oxidative stress. They also found elevated levels of neurotrophic factors.
Zigmond concedes that more studies are required to say with absolute certainty that exercise and social interactions can provide neuroprotection to humans with PD. But he is encouraged by a number of promising epidemiological studies. “What people have begun to do is to look backwards,” Zigmond states. “And what they are finding is that people who routinely exercise are less likely to get Parkinson’s than people who don’t.”

Ancient Genes in a Modern World
Zigmond contends that we can better understand neurodegenerative diseases and indeed other lifestyle maladies such as diabetes and cardiovascular disease if we viewed them through the lens of evolutionary psychology. Up until about a few thousand years ago, our ancestors lived as hunter-gatherers in an environment that was unpredictable and often hostile. According to him, this meant that they spent enormous energy in hunting down animals, collecting wild plants and escaping from predators. It also meant that they spent a considerable amount of time engaging in social interactions since they had to cooperate with other members of the group. “People stuck together in small groups in which they shared food, protected each other, and raised children.”
Today, however, we live radically different lives in a radically different environment. This, Zigmond says, is a problem because biologically we are still almost identical to our ancestors who roamed the savannas of Africa. “We have more or less the same genes because evolution proceeds very, very slowly,” he points out. The mismatch, he believes, has consequences. “We sleep less, we exercise less, we eat all the time, we interact less with others. As a result, our ‘healthspan’ has decreased. We suffer from more non-communicable diseases – heart disease, lung disease, neurodegenerative diseases, clinical depression. It also has a huge financial and emotional cost,” he laments.
To Zigmond, the way out of our current predicament is straightforward: exercise more, eat better, and spend quality time with family and friends. “We don’t have to have these diseases in many cases. And when they do emerge, they will emerge at a later age than what we are seeing now,” he concludes.




