G Padmanaban has spent decades trying to understand the lifecycle of the malaria parasite and develop a cure

A few years ago, researchers from Michigan State University tracking malaria in Malawi made a gut-wrenching discovery. The reason why many children with cerebral malaria were dying was because their brain was swelling up so much that it pushed out through the bottom of the skull and squeezed their brain stem, stopping their breathing.
A devastating complication arising from malarial infection, cerebral malaria kills thousands of children under the age of five in Africa, and leaves survivors suffering from long-term neurological problems.
The only effective treatment currently available for such severe forms of malaria is artemisinin – a compound found in a herb used for over 2,000 years in Chinese traditional medicine. It was first extracted in 1972 – an effort that earned a Nobel Prize – and its subsequent widespread use saved millions of lives. In order to prevent the malaria parasite from becoming resistant to artemisinin, the World Health Organisation declared in 2006 that it should not be given alone, and that combination therapies should be developed with other antimalarial drugs or compounds.
To G Padmanaban, who had by then been studying the disease for decades, this was an exciting proposition because he was already working on just that – trying to combine artemisinin with curcumin, another molecule having ancient roots.
By 1998, he had formally retired as the Director of IISc, but continued working as an Honorary Professor at the Department of Biochemistry. After delving deep into literature on the medicinal properties of curcumin, he became increasingly convinced that it had enormous potential for treating a variety of diseases.
Working with PN Rangarajan, now the Chair of the department, Padmanaban found that three oral doses of curcumin along with a single injection of an artemisinin derivative prevented malaria relapse in infected mice in the lab, and boosted their chances of survival. They also showed that the combination staved off neurological symptoms linked to cerebral malaria and completely cured infected mice.
Buoyed by these results, Padmanaban was eager to take the combination therapy to the market. He filed a patent in both India and the US. He also urged the Drug Controller General of India (DGCI) to carry out clinical trials to test its efficacy in patients.
But he hit roadblocks on both fronts. The National Biodiversity Authority (NBA) pulled him up for apparently not seeking clearance before filing the US patent because turmeric – the spice from which curcumin is usually extracted – is considered an endangered species. His arguments that they had not used turmeric extract but a commercially sold chemical form of curcumin fell on deaf ears. As the penalty could end up being a fine of Rs 10 lakh and a jail term, Padmanaban’s patent attorney even advised him to withdraw the sanctioned US patent.
The DGCI, for its part, dragged its feet on the clinical trial application for over a decade. Last year, after a relentless battle, it finally gave approval for the trials to be carried out in two hospitals in Chhattisgarh and Rourkela by the National Institute of Malaria Research (NIMR), New Delhi. A tablet made from curcumin and an artemisinin derivative called artesunate was to be tested in patients with simple malaria. The clinical trial project was approved by the Department of Biotechnology. Everything was set. Then, COVID-19 struck. Now, NIMR is waiting for the hospitals to return to their normal routine.
“Sometimes I feel like [the mythological king] Bhagiratha trying to bring the river Ganges down to Earth,” chuckles Padmanaban, now 82.
The haem connection
By the time Padmanaban applied to IISc for a PhD in late 1960, all formal admissions for that year had closed. Luckily, he was selected as a Junior Research Fellow in a sponsored project under PS Sarma at the Department of Biochemistry. Sarma’s lab was working on kesari dal, a controversial pulse whose excess consumption was linked to neurological disorders. Padmanaban was able to isolate the amino acid from kesari dal that turned out to be a neurotoxin. But then, he was asked to work on trace element metabolism as that was the goal of the sponsored project.
While carrying out experiments on a type of mould under iron-starved conditions, he stumbled upon the synthesis of haem, an iron-containing molecule also found in haemoglobin, the oxygen-carrying protein of red blood cells. He developed a keen interest in this molecule, which would serve him well years later.
After completing his PhD in 1966, he continued working in Sarma’s lab, giving up the chance to go abroad. In 1969, he was appointed as an assistant professor in the same department. “Everybody was working on molecular biology in prokaryotes. But I wanted to work on animals, and humans, which was considered a ‘black box’,” he says. He learnt all the techniques needed in the lab of Murray Rabinowitz at the University of Chicago, which he visited 10 times between 1973 and 1986.
Around the early 1980s, the Swedish pharmaceutical firm Astra – now AstraZeneca – approached Padmanaban and asked for his help to set up a research centre in Bangalore. With support from the Department of Science and Technology (DST), Government of India, the company acquired a building close to IISc campus. Padmanaban opened up his own lab to the Astra team. “It was one of those old labs, with cockroaches and all,” he reminisces. “But still research went on.” He also convinced about half a dozen students, who were planning to go abroad for their PhD, to stay back and work on joint projects with Astra.
(Image courtesy: Genome Research Limited/Wellcome Trust)
The company was keen on carrying out research on infectious diseases in India and provided generous funding. “We built the entire north wing of the department with Astra money,” he recalls. Spurred by their interest, he began projects on four diseases, one of which was malaria. Eventually Astra moved out to Hebbal in the city and was later shut down, but Padmanaban continued working on malaria, which soon consumed his entire attention.
“It was the haem connection,” he explains. The malaria parasite, Plasmodium, breaks down the haemoglobin in our red blood cells to release haem and amino acids, which are vital for its survival. But free haem can kill the parasite, so it converts the haem into a safer form called haemozoin. Drugs such as chloroquine work by blocking this conversion.
However, as Padmanaban discovered, the parasite was also able to synthesise haem by itself. If it was getting plenty of haem from our blood, why would it need to make its own? “We were more or less the only group in the world looking at this question,” he says.
Shortly after Padmanaban formally retired, Arun Nagaraj, now a scientist at the Institute of Life Sciences, Bhubaneswar, joined him and continued investigations into the parasite’s life cycle, using lab mice infected with a related species called Plasmodium berghei.
First, they tried to see if knocking out genes involved in haem synthesis stopped the parasite from growing. “But it didn’t do anything. The parasite continued to grow merrily,” explains Padmanaban.
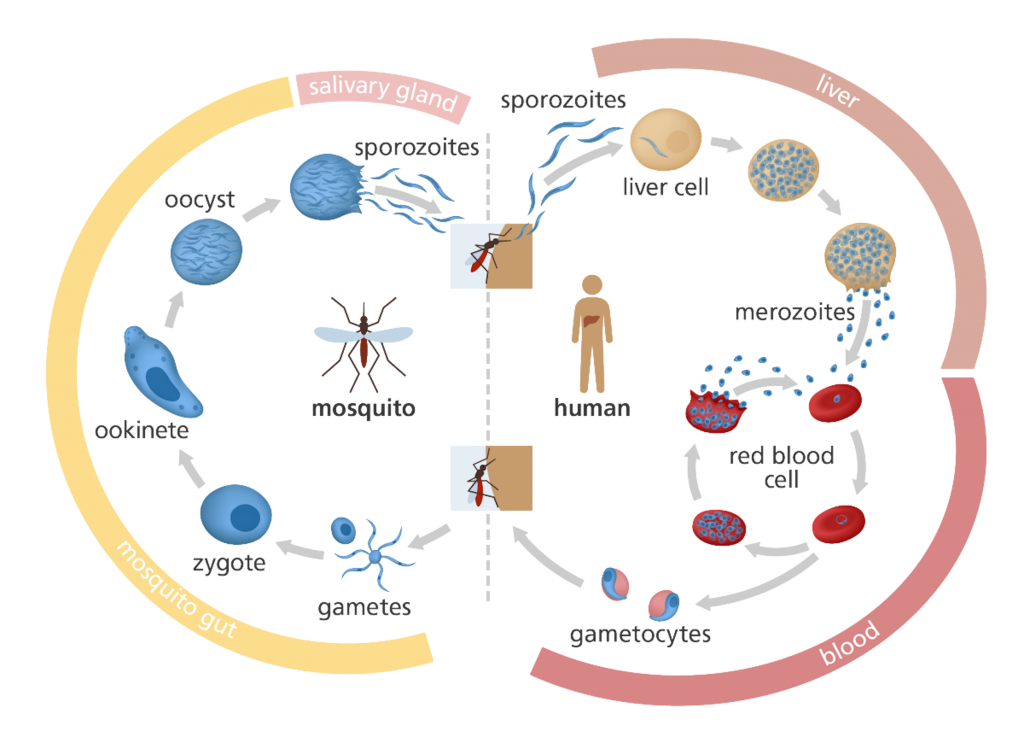
Then, they wondered if they were looking in the wrong place. So far they were only looking at one host – the human. But the parasite’s life actually cycles between the human and another host – the female Anopheles mosquito. When the mosquito bites us, it injects larvae-like forms of the parasites (sporozoites) into our body. Inside our liver, these sporozoites grow and transform into merozoites, which burst out into the bloodstream. These merozoites then develop into male and female sexual forms, which are sucked back into another mosquito that bites us the next time. Inside the newly infected mosquito, the sexual forms fuse to eventually give rise to more sporozoites, thus completing the cycle.
Perhaps the parasite didn’t need to make haem to grow inside the human red blood cells, but needed it inside the mosquito.
To test this theory, the team had to breed infected mosquitoes, slice them open and study them. “At that time, we didn’t have an insectary to grow the mosquitoes,” says Nagaraj. He had to travel 40 km from IISc to an ICMR lab close to the airport, where they could breed the infected mosquitoes, and carry them back to campus to test them on mice models. Each experiment would take 40 days, and frequent power disruptions in the ICMR lab meant entire experiments had to be scrapped, because the mosquitoes needed steady environmental conditions to survive.
Nagaraj had to travel 40 km to an ICMR lab where they could breed the infected mosquitoes, and carry them back to campus to test them on mice models
Their perseverance eventually paid off. In 2013, they published a study showing that the parasite absolutely needs to make its own haem inside the mosquito and in the human liver. Parasites that could not make haem were unable to sexually reproduce. “They can survive in the [human] blood stage, but when they go back into the mosquito, you will not find development of sporozoites,” explains Nagaraj.
But the mystery
wasn’t completely solved yet. Yes, the parasite needed to make haem inside the
mosquito and yes, it also had access to haem from haemoglobin. Then why would
it continue to make haem inside human blood cells?
“Finally, we have an answer for that now; we have not yet published the data,” says Nagaraj. “The parasite makes haem to sustain virulence.” He says that they have also identified a drug already in use that can be repurposed to block haem synthesis and abolish parasite virulence.

Curcumin as a cure
The problem with developing new drugs to treat malaria is that the parasite can quickly become resistant to them. Its genetic material is rich in a specific DNA base pair combination that makes it unstable and easily mutable. “You develop a drug or vaccine, it will develop resistance. If [the drug or vaccine] works one year, it may not work the next year. And what works in India may not work in Africa,” explains Padmanaban.
Plasmodium falciparum, the deadliest malarial parasite species, has already become resistant to many of the partner drugs used with artemisinin. Alarming reports of artemisinin resistance are also emerging across Southeast Asia, and more recently in Africa. “There is no alternative drug, really speaking, although there are drugs in the pipeline, in clinical trials,” says Padmanaban.
This is where he believes a molecule like curcumin can make a difference, at least as an adjunct drug. It is not a newly developed compound, but a food molecule that has been consumed by humans for millennia. The parasite is therefore unlikely to become resistant to it, he says.
Initially when his team tested the effect of curcumin, they found that it could only delay parasite growth in culture. Then, they decided to combine it with an artemisinin derivative called arteether and the results were more encouraging. Giving three oral doses of curcumin with a single shot of arteether was especially helpful in preventing the infection from relapsing, a common problem with some drug combinations. Weeks after giving the combination therapy, the team could detect antimalarial antibodies circulating in the blood of infected mice.
In another experiment, this time on cerebral malaria, the team found that curcumin alone could prevent the onset of neurological symptoms. It prevented the breakdown of the blood-brain barrier – which regulates the flow of molecules from the blood into the brain – and clumping of red blood cells in the brain, and therefore reduced inflammation. The mice still ended up dying because of anaemia. But when curcumin was given along with arteether, the combination once again saved the mice from death.
Inspite of these promising results, Padmanaban had a hard time convincing the DGCI to approve the clinical trials. Their argument, he says, was that a naturally occurring molecule like curcumin should not be combined with a chemical artemisinin derivative.
Another issue, as Padmanaban writes in his book Doing Science in India: My Second Innings, is that curcumin “does not act like a drug in a way that is understood by medicinal chemists.” Some believe that it won’t be as effective as other drugs because it gets broken down within minutes of entering the bloodstream. “Then, how is it that the protective effects of curcumin are seen after several days?” he writes. “We could detect copious amounts of anti-parasite antibodies in the blood at the time of parasite recrudescence [recurrence] only in curcumin-treated animals.” He speculates that curcumin likely activates memory cells of the immune system to respond to the parasite, although those mechanisms are yet to be understood.

Despite the delays due to COVID-19, Padmanaban is optimistic about the success of the clinical trials testing the combination therapy. “This will be the first powered trial to establish that it works,” he says. “It will take another 4-5 years. But it will be worth it.” His patent battle also thankfully ended last year. “The government said that the NBA should reinvent itself and settle all cases before 2019. In that process, ours was dropped.”




