India is the world’s tuberculosis capital, accounting for 27 percent of the 10 million patients globally. Understanding how drug resistance emerges and improving vaccine efficacy are key to fighting this disease.
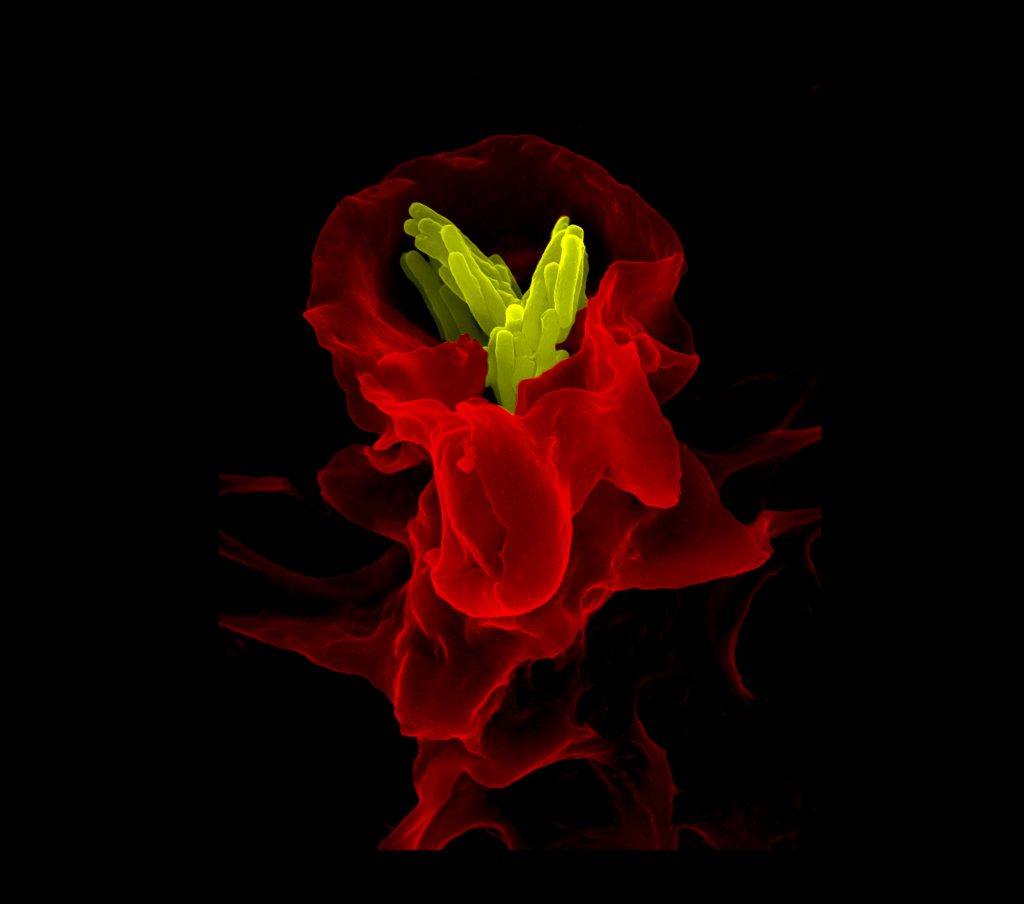
Macrophage (red) engulfing tuberculosis bacteria (yellow), taken with ZEISS FE-SEM (Image courtesy: Volker Brinkmann)
In 1890, Robert Koch believed he was going to take the world by storm. Again.
Eight years earlier, the German physician had made a stunning discovery. The “great white plague” that had ravaged the continent for centuries was not caused by “bad air” or genes, but by a microscopic killer: Mycobacterium tuberculosis.
On 6 August 1890, Koch made a sensational announcement. He had found a remedy for tuberculosis: a mysterious formulation he called tuberculin.
Excitement and hope spread. Patients and journalists flocked to Berlin. Among them was a little-known English physician and soon-to-be famous author named Arthur Conan Doyle.
According to reports, Conan Doyle was unable to meet the scientist or attend his lectures. Yet, he managed to visit patients who had been given tuberculin, and browse through notes from Koch’s lectures. Unlike others, he was unconvinced that the remedy was working. In a letter to The Daily Telegraph, he singled out the fact that tuberculin was not fighting the bacterium itself, only removing traces of infected tissue.
Soon, it became clear that tuberculin wasn’t making patients better; in fact, some were getting worse. Criticism mounted, and Koch was eventually forced to publicly admit that his remedy was a failure.
More than a century after Koch’s debacle, TB is now considered fully curable, thanks to the development of powerful antibiotics such as isoniazid and rifampicin. But the fight is far from over.
Battling drug resistance
Although Koch discovered the bacterium only in 1882, some forms of TB infection have existed for millennia. The oldest known case is from a 9,000-year-old skeleton of a woman and her baby found off the coast of Israel. Signs of infection have also been spotted in 5,000-year-old Egyptian mummies.
The fact that the bacterium has managed to escape from being eradicated for so long has stumped scientists.
“It is a very successful pathogen,” says Nagasuma Chandra, Professor at the Department of Biochemistry, IISc. “It has been able to survive the immune system at various stages, and has survived people’s efforts to combat it.”
For one, the bacterium can stay dormant inside a host for decades. Once it enters the lungs, it can take on either a Mr. Hyde-like aggressive form, or a mild-mannered Dr. Jekyll-like form. While Hyde goes on a rampage, multiplying aggressively, attacking immune cells, and spreading to other organs, Jekyll stays quietly hidden inside the immune cells for years, waiting to transform one day. About a quarter of the world’s population is thought to have the latter form of infection, called latent TB.
What makes the bacterium more dangerous, however, is its growing ability to resist being killed by antibiotics.
What makes the bacterium more dangerous, however, is its growing ability to resist being killed by antibiotics
For decades, a potent and safe antibiotic called isoniazid has been used as the frontline drug to treat TB, in combination with two or three others. But bacterial strains resistant to isoniazid are fast emerging.
A few years ago, Chandra’s lab started investigating how this drug resistance arises, using a lab-grown isoniazid-resistant strain of a close relative called Mycobacterium smegmatis. Instead of looking at only a few pathways or proteins, they mapped entire networks of proteins inside the bacterium, and used computational tools to tease out which mechanisms were activated in the resistant strain. This is akin to building a roadmap of the city to track which roads show unusual activity when there is a roadblock.
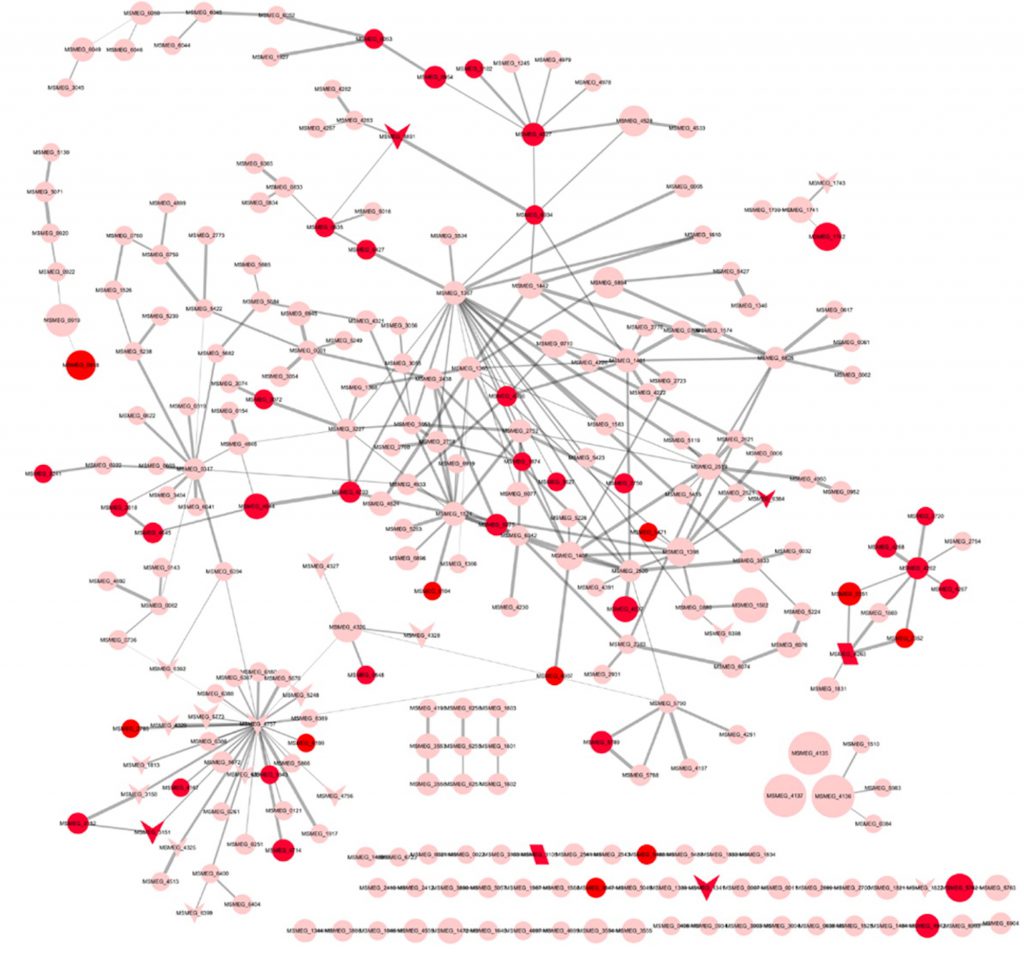
Emerging drug resistance: Mapping protein interaction networks (Image courtesy: Jyoti Padiapu et al./ACS Infectious Diseases)
“We found that multiple mechanisms are active simultaneously,” says Chandra. Most of them were involved in fighting oxidative stress induced by the drug – they were helping the bacterium defend itself against toxic oxidant molecules. With multiple mechanisms turned on, the bacterium’s threshold to handle stress had gone up.
Once the researchers learnt this, all they had to do was raise the oxidative stress beyond this threshold, using the right type of drugs. “Let us say that the bacterium has twenty soldiers that are ready to fight the stress. All twenty are now deployed. If you give additional stress, there are no soldiers left,” says Chandra.
Working with Amit Singh, Associate Professor at the Department of Microbiology and Cell Biology, IISc, Chandra’s lab screened existing drugs that are known to induce oxidative stress and picked three: ebselen, vancomycin and phenylarsine oxide. Combining isoniazid with any one of these dramatically increased the bacterium’s vulnerability to the antibiotic.
The researchers are now beginning to test the vancomycin-isoniazid combination in animal models. “Vancomycin is already used in the clinic for Methicillin-resistant Staphylococcus aureus (MRSA) infection,” says Chandra. “We thought it would be easy to go to clinical trials.”
Purging the persisters
Even before Koch’s time, what fueled the TB rampage across Europe was the overcrowding, poor sanitation and malnutrition that mushroomed during the industrial revolution. Today, people in poor living conditions continue to be at a greater risk of infection. They also suffer the most financially because the treatment can be long-drawn-out and expensive.
“If a farmer or a daily wage worker gets TB, and they have to take 2-4 pills everyday for 6-9 months, that is a big burden on their resources,” says Singh. There’s another danger: if they stop taking the drugs abruptly, the bacteria that are not yet killed will likely grow resistant.
Singh’s lab has been trying to cut down this treatment time. Recently, they zeroed in on a subset of TB bacteria displaying tolerance to anti-TB drugs.
When TB bacteria enter the body, they are engulfed by white blood cells called macrophages dispatched by the body’s immune system. Inside the macrophages, the bacteria are walled off and bombarded with digestive enzymes and lethal molecules. Most of them die. But a subset – about 30 percent – somehow survive inside the macrophages, and grow tolerant to drugs. Getting rid of these tolerant bacteria early on could help reduce the treatment time.
“What is so different in these 30 percent?” says Singh. “How are they generated? Are they generated in response to the macrophage environment? If so, what is that environment which allows them to become drug-tolerant?”
Last year, Singh’s lab had a breakthrough. They found that the macrophages containing these tolerant bacteria were different from others: their internal environment was more acidic.
An acidic macrophage environment can induce redox stress in the bacterium by freeing up toxic metals such as iron and copper. Freely available metals can seep into the bacterial cell causing a metal overload and unleashing toxic free-radicals. To survive, the bacterium turns on mechanisms that help it fight the stress caused by these free-radicals. The same mechanisms can help it become drug-tolerant, says Singh.
To reduce the acidity, the researchers gave infected mice chloroquine, a weak base, along with isoniazid. “The results were dramatic,” says Singh. Within eight weeks, the lungs of infected mice were completely clear of bacteria.
Chloroquine is a safe drug that is already being used to treat malaria. “If it is given together with anti-TB drugs, we might be able to reduce the therapy time from 6 months to 4,” says Singh.
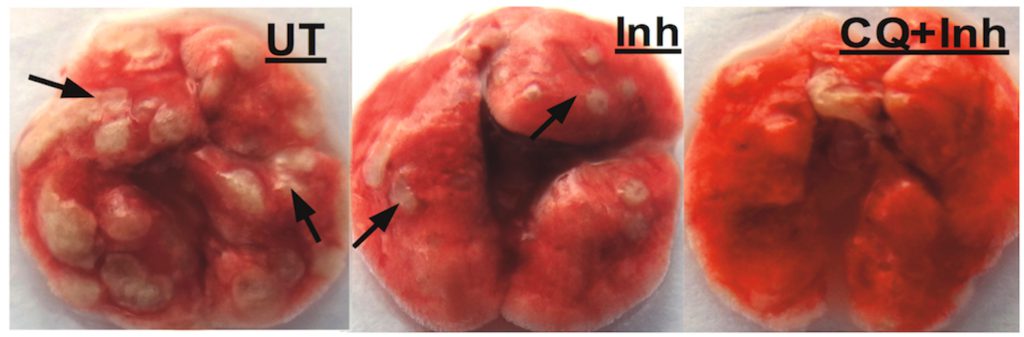
Lungs from untreated infected mice, infected mice treated with Isoniazid alone, treated with isoniazid (Inh) + chloroquine (CQ). Arrows indicate presence of granulomas, lesions formed by immune cell aggregates in lungs of TB patients (Image: Richa Mishra)
Reviving BCG
If Koch’s discovery of M. tuberculosis was a turning point in TB history, the development of the first and only vaccine was another.
Spurred by the success of the smallpox vaccine, French scientists Albert Calmette and Camille Guerin took a deadly strain of Mycobacterium bovis – responsible for TB in cows – and spent 13 years weakening it until it could no longer cause disease. In 1921, their new vaccine, called Bacille Calmette-Guerin (BCG), was given to humans for the first time. BCG made its way to India in 1948, and is now part of the dozen vaccines mandated under the government’s Universal Immunisation Programme.
The vaccine is effective in protecting infants from tuberculosis meningitis and miliary disease, but its protection wears off after a few years.
“BCG, while extremely safe, is a poor vaccine, with very poor efficacy against adult pulmonary tuberculosis. By the time a [vaccinated] person turns 15, they are just as prone to getting TB as an unvaccinated person,” says S Vijaya, Professor at the Department of Microbiology and Cell Biology, IISc.
In recent years, researchers have begun to ask if giving BCG once again in adulthood might help. A trial of 990 individuals in South Africa published last year offers some answers. It found that revaccination can not only reduce the chance of infection, but also potentially eliminate bacteria in those with latent TB. “There is now a lot of interest in trying to see if revaccination of young adults in the age group of 18-22 years is capable of boosting anti-TB immunity,” says Annapurna Vyakarnam, Visiting Scientist and Ramalingaswami Fellow at the Centre for Infectious Diseases Research, IISc.
Researchers have begun to ask if giving BCG once again in adulthood might help
To see if revaccination could work in India, Vyakarnam and her colleagues recruited 200 people, most of whom were young nursing students at an old TB sanatorium in the city of Madanapalle, Andhra Pradesh. All of them had been given BCG at birth. They were split into two groups: those who had latent TB, and those who did not carry the bacteria. Within each group, half were revaccinated with BCG. Blood samples were extracted and analyzed over nine months, and detailed flow cytometry investigations were carried out to check for the presence of up to 256 immune cell subsets.
What the researchers found was encouraging. Revaccinated individuals had twice as many immune cells belonging to specific subsets called CD4 and CD8 T-cells. These cells produce signalling proteins called cytokines that rally the body’s immune responses against TB. Revaccination was also found to boost the numbers of innate immune cells that form the first line of defense against TB.
“We are able to demonstrate for the first time that revaccination is immunogenic in the Indian context. It induces the right type of immune cells,” says Vyakarnam. “What our study proved in addition was that it is putatively safe to vaccinate individuals who aren’t infected…[it is] not going to accelerate disease in the individual.”
Developing new vaccines
Given how poor BCG is at preventing TB in adults, can new vaccines be developed that offer longer lasting protection?
The answer, many believe, lies in understanding how some people who have been exposed to TB for years – such as hospital staff or caregivers – continue to remain healthy. “These individuals, we know, have naturally acquired protective immunity to the disease,” says Vijaya.
A few years ago, her team found that the immune system in these individuals was explicitly targeting certain proteins that the bacterium produced, which the diseased individuals were unable to. Two proteins called Rv1860 and Rv3881c were found to trigger a particularly strong immune response in those who were healthy. Vijaya’s team took the genes encoding these proteins, added it to BCG, and gave this to guinea pigs that were then infected with TB.
“We showed that this improved BCG is able to control the disease much better than the parent BCG,” says Vijaya. “In one of our experiments, we found that a couple of animals actually had sterilising immunity…[the added genes] were improving BCG to an extent that the bacterial load was below limits of detection.”
But translating these lab results to a commercial vaccine is not easy, Vijaya points out. “To take it to human clinical trials, you need large amounts of funding…in India, nobody has the political will or the technical expertise.”

S Vijaya showing the Biosafety Level-3 facility, which she helped set up at IISc, to former Director P Balaram and former Associate Director N Balakrishnan. A major part of tuberculosis research at the Institute is carried out here (Photo courtesy: S Vijaya)
Some steps are, however, being taken to improve the current state of vaccine development. The Indian Council of Medical Research recently launched an extensive clinical trial of two vaccine candidates – a German BCG recombinant and a leprosy vaccine that produces proteins similar to the TB bacterium. These are being tested in more than 12,000 healthy household contacts of TB patients. In addition, the government is pumping Rs 12,000 crore into its National Strategic Plan for TB Elimination, to ensure improved access to quality diagnosis, treatment, and support. It also claims that it will eliminate TB in India by 2025.




